Abstract
Objective
It had been previously shown that patients who receive neoadjuvant systemic therapy (NST) are more likely to undergo breast-conserving therapy (BCT) than those who have primary surgery. However, the frequency with which patients who are not BCT-eligible prior to NST convert to BCT-eligible with treatment is unknown. To document this conversion rate in a subset of patients expected to have a high clinical response rate to NST, we studied surgical assessment and management of patients enrolled on a randomized neoadjuvant trial for stage II–III HER2-positive breast cancer (HER2 + BC)(CALGB 40601).
Methods
The treating surgeon assessed BCT candidacy based on clinico-radiographic criteria both before and after NST. Definitive breast surgical management was at surgeon and patient discretion. We sought to determine (1) the conversion rate from BCT-ineligible to BCT-eligible (2) the percentage of BCT-eligible patients who chose breast conservation, and (3) the rate of successful BCT. We also evaluated surgeon-determined factors for BCT-ineligibility and the correlation between BCT eligibility and pathologic complete response (pCR).
Results
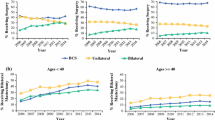
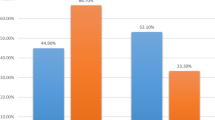
Of 292 patients with pre- and post-NST surgical assessments, 59 % were non-BCT candidates at baseline. Of the 43 % of these patients who converted with NST, 67 % opted for BCT, with an 80 % success rate. NST increased the BCT-eligible rate from 41 to 64 %. Common factors cited for BCT-ineligibility prior to NST including tumor size (56 %) and probable poor cosmetic outcome (26 %) were reduced by 67 and 75 %, respectively, with treatment, while multicentricity, the second most common factor (33 %), fell by only 16 %. Since 23 % of the BCT-eligible patients chose mastectomy, BCT was the final surgical procedure in just 40 % of the patients. Patients considered BCT-eligible both at baseline and after NST had a pCR rate of 55 %, while patients who were BCT-ineligible prior to NST had the same pCR rate (44 %) whether they converted to BCT-eligible or not.
Conclusions
Many patients with HER2 + BC deemed ineligible for BCT at baseline can be converted to BCT-eligible with NST; excluding patients with multicentric disease substantially increases that percentage. In converted patients who opt for BCT, the success rate is similar to that of patients considered BCT-eligible at baseline. Whether a BCT-ineligible patient converts to BCT eligibility or not does not appear to affect the likelihood of achieving a pCR. Despite the efficacy of NST in this patient cohort, only 40 % of patients had successful BCT; further research into why BCT-eligible patients often opt for mastectomy is needed.



Similar content being viewed by others
References
Barry PA, Schiavon G (2015) Primary systemic treatment in the management of operable breast cancer: best surgical approach for diagnosis, biological evaluation, and research. J Natl Cancer Inst Monogr 2015(51):4–8
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97(3):188–194
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol Off J Am Soc Clin Oncol 26(5):778–785
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol Off J Am Soc Clin Oncol 19(22):4224–4237
Golshan M, Cirrincione CT, Sikov WM et al (2015) Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 262(3):434–439
Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, Ollila DW, Krop IE, Henry NL, Weckstein DJ, Anders CK, Singh B, Hoadley KA, Iglesia M, Cheang MC, Perou CM, Winer EP, Hudis CA (2016) Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol 34(6):542–549
Criscitiello C, Azim HA, de Azambuja E, Rubio IT (2014) Factors affecting surgical management following neoadjuvant therapy in patients with primary HER2-positive breast cancer: results from the NeoALTTO phase III trial. Ann Oncol Off J Eur Soc Med Oncol ESMO 25(4):910–911
von Minckwitz G, Schneeweiss A, Loibl S et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15(7):747–756
Debled M, MacGrogan G, Breton-Callu C et al (2015) Surgery following neoadjuvant chemotherapy for HER2-positive locally advanced breast cancer. Time to reconsider the standard attitude. Eur J Cancer 51(6):697–704
Holmes D, Colfry A, Czerniecki B et al (2015) Performance and practice guideline for the use of neoadjuvant systemic therapy in the management of breast cancer. Ann Surg Oncol 22(10):3184–3190
McGuire KP, Hwang ES, Cantor A et al (2015) Surgical patterns of care in patients with invasive breast cancer treated with neoadjuvant systemic therapy and breast magnetic resonance imaging: results of a secondary analysis of TBCRC 017. Ann Surg Oncol 22(1):75–81
Caudle AS, Yu TK, Tucker SL et al (2012) Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res BCR 14(3):R83
Cureton EL, Yau C, Alvarado MD et al (2014) Local recurrence rates are low in high-risk neoadjuvant breast cancer in the I-SPY 1 Trial (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol 21(9):2889–2896
Kummel S, Holtschmidt J, Loibl S (2014) Surgical treatment of primary breast cancer in the neoadjuvant setting. Br J Surg 101(8):912–924
Rippy EE, Ainsworth R, Sathananthan D, Kollias J, Bochner M, Whitfield R (2014) Influences on decision for mastectomy in patients eligible for breast conserving surgery. Breast 23(3):273–278
Acknowledgment
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), as well as U10CA180888 (to SWOG). Also supported in part by funds from the Breast Cancer Research Foundation, Genentech, and GlaxoSmithKline. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Golshan, M., Cirrincione, C.T., Sikov, W.M. et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II–III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat 160, 297–304 (2016). https://doi.org/10.1007/s10549-016-4006-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4006-6