Abstract
Phase I pharmacokinetic (PK) study assessed circulating estrogens in breast cancer (BC) patients on a non-steroidal aromatase inhibitor (NSAI) with vaginal atrophy using vaginal ultra-low-dose 0.03 mg estriol (E3) and Lactobacillus combination vaginal tablets (Gynoflor®). 16 women on NSAI with severe vaginal atrophy applied a daily vaginal tablet of Gynoflor® for 28 days followed by a maintenance therapy of 3 tablets weekly for 8 weeks. Primary outcomes were serum concentrations and PK of E3, estradiol (E2), and estrone (E1) using highly sensitive gas chromatography–mass spectrometry. Secondary outcomes were clinical measures for efficacy and side effects; microscopic changes in vaginal epithelium and microflora; and changes in serum FSH, LH, and sex hormone-binding globulin. Compared with baseline, serum E1 and E2 did not increase in any of the women at any time following vaginal application. Serum E3 transiently increased after the first application in 15 of 16 women, with a maximum of 168 pg/ml 2–3 h post-insertion. After 4 weeks, serum E3 was slightly increased in 8 women with a maximum of 44 pg/ml. The vaginal atrophy resolved or improved in all women. The product was well tolerated, and discontinuation of therapy was not observed. The low-dose 0.03 mg E3 and Lactobacillus acidophilus vaginal tablets application in postmenopausal BC patients during AI treatment suffering from vaginal atrophy lead to small and transient increases in serum E3, but not E1 or E2, and therefore can be considered as safe and efficacious for treatment of atrophic vaginitis in BC patients taking NSAIs.
Similar content being viewed by others
Introduction
Estrogen deprivation with oral aromatase inhibitors (AI) is an established therapy in postmenopausal women with an estrogen (ER) and/or progesterone receptor (PR) positive breast cancer (BC) [1]. Although they improve survival, AIs worsen or induce vaginal atrophy, dryness, and dyspareunia in most women [2]. Some develop atrophic vaginitis, a subtype of aerobic vaginitis (AV) [3]. These side effects dramatically reduce the quality of life (QoL) and hamper compliance which affects patient survival [4]. As more women using AI therapy are surviving BC for many years, these side effects become a major challenge for both patients and their physicians [5, 6].
Vaginal estrogen application is the most effective therapy to alleviate these symptoms [4, 7, 8] and is clearly more efficacious than non-hormonal therapies [9–11]. However, vaginal administration of any dose of estradiol (E2) in AI users increases serum levels of E21 [3–14] [15–18]. Therefore, most authors see such treatment as a potential danger in women with a history of BC women as systemic absorption of estrogen can stimulate the growth of breast cancer cells [5, 6, 19–22]. Thus, since safety is a major issue, only less potent estrogens should be considered for vaginal treatment. E3, a less potent estrogen than E2, the vaginal application of 1 mg in postmenopausal women with vaginal atrophy did not increase serum levels at 2 weeks, 3 months, and 6 months as compared to controls, and endometrial biopsies showed no proliferation [23].
Gynoflor® contains 10 [8] viable lyophilized Lactobacillus acidophilus (L. acidophilus KS400) bacteria and 0.03 mg E3, which is a 16–32 times lower dose than in conventional E3 vaginal preparations (0.5–1 mg). This product has been proven to be safe and efficacious in restoration of the disturbed vaginal flora [24, 25] and in treatment of postmenopausal atrophic vaginitis [26–29]. Former data indicate that application of one tablet of Gynoflor® daily in healthy postmenopausal women with vaginal atrophy doubles C max of E3 of compared with baseline level, but still within the postmenopausal range at day 1; whereas at day 12, C max compared with baselevel was not increased at all [30].
This phase I pharmacokinetic study primarily assessed circulating estrogens and efficacy after vaginal ultra-low-dose 0.03 mg estriol (E3) and L. acidophilus combination vaginal tablets (Gynoflor®) in BC patients on a NSAI.
Subjects and methods
This was an open label bicentric phase I pharmacokinetic (PK) study, in 16 postmenopausal women on a NSAIs and suffering from symptomatic vaginal atrophy. This clinical trial was conducted at two centers: one in Belgium and one in Germany, and patients were included from April 2011 until July 2012. The study was approved by both the Ethical Committees (IEC) and the national authorities as appropriate (EudraCT No: 2010-022007-22) and all patients signed informed consent before any study action was taken, according to GCP and the declaration of Helsinki. Hormone analysis was performed by Nuvisan GmbH, Germany; vaginal smear analysis was made by Femicare vzw, Belgium, and a PK statistics—by Arlenda SA, Belgium. This report complies with the CONSORT guidelines.
Included women were postmenopausal at an age of 52 years or more or ≥46 years after bilateral oophorectomy with cessation of menses for at least 12 months and started AI at least 6 months ago. Furthermore, in women after hysterectomy with intact ovaries, FSH levels had to be above 30 IU/l. Additional criteria were the presence of clinical symptoms of vaginal atrophy, vaginal pH > 5.0, and a Karnofsky score ≥ 80 %.
Main exclusion criteria were use of any other sex hormones or phytoestrogens 6 months before or during the study, use of any other vaginal medication, use of anti-infectives, and use of steroidal AIs, sexually transmitted infections or malignant or pre-cancerous conditions. Women with a BMI lower than 18.5 or higher than 30 were also excluded.
Gynoflor® vaginal tablets (100 million viable L. acidophilus KS400 and 0.03 mg E3) were supplied by Medinova AG, Switzerland. Recruited women underwent an initial treatment for 4 weeks (1 vaginal tablet inserted daily deep into the vagina before sleep and on PK testing days—at entry and at visit after 4 weeks—early in the morning) followed by maintenance therapy (3 vaginal tablets weekly with one every second day) for 8 weeks.
The primary aim was to determine the absorption and PK parameters of E3 and its influence on the serum concentrations of E2 and E1 during initial daily therapy. Secondary goals were to test serum levels of E3, FSH, luteinizing hormone (LH), and sex hormone-binding globulin (SHBG), and also to evaluate clinical symptoms and changes in the physiological status of the vaginal epithelium and microflora, to compare the treatment success during initial and maintenance therapy, and to assess the safety profile.
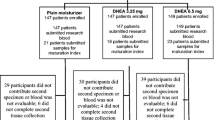
Clinical examinations were performed at screening (S = week–1), at entry (E = Day 0), and at days 14 (C1 = week 2), 28 (C2 = week 4), 56 (C3 = week 8), and 84 (C4 = week 12) to assess hormone levels, efficacy, and safety (Fig. 1).
Study design. At screening, eligible patients were included. At Entry visit, an initial tablet of g-Gynoflor® was introduced and a PK study to detect serum estrogens over a 24 h period was performed (Visit E). Patients were checked at 2 weeks for serum estrogen levels, vaginal responses, and side effects (1). At day 28 (Visit C2), 56 (Visit C3), and 84 (Visit C4), the same variables were checked, but on day 28 (Visit C2) another 24 h PK study for serum estrogen dynamics was performed. Between visit E and visit C2 patients applied 1 vaginal tablet daily (Initial therapy phase), whereas after visit 2 Gynoflor® was used every second day (Maintenance phase)
Multiple blood samples for PK parameters were taken at visit E and C2 at 0.5 h before and 0.5, 1, 2, 3, 4, 6, 8, and 24 h after test drug application. In addition, at each visit, samples were taken for testing of trough serum levels of E3, E2, and E1 and concentrations of FSH, LH, and SHBG.
Estrogens were analyzed using a highly sensitive gas chromatography–mass spectrometry (GC/MS) method (validated according to FDA Guidance for industry). After extracting, cleaning-up, and derivation of 1 ml of serum, 1–2 μl of sample was injected into the GC/MS system. Measurements were performed in the chemical ionisation mode with negative ions using ammonia as reagent gas. The lower limits of quantitation (LLOQ) were 10.00 pg/ml for E3, 1.00 pg/ml for E2, and 2.00 pg/ml for E1. The coefficient of variation (CV, intra-assay variation) was 2.0 % for E3 (calibration range (CR) 10.00–500.00 pg/), 4.2 % for E2 (CR 1.00–150.00 pg/ml), and 3.4 % for E1 (CR 2.00–300.00 pg/ml). FSH, LH, and SHBG measurements were performed using automated immunoassay analyser system Access® by Beckman Coulter Inc., USA following basic principle of a competitive immunoenzymatic binding assay.
The primary efficacy parameters were serum concentrations of E3, E2, and E1, and E3 PK parameters on days 0 (visit E) and 28 (visit C2). The trough E3, E2, and E1 levels were pre-dose concentrations (−0.5 h) at control visits. Concentrations below the LLOQ were considered as zero in descriptive statistics. The area under the curve from administration to the last measured concentration (AUC0–24) was calculated by linear trapezoidal integration. The highest measured concentrations were reported as C max. Peak times evaluated as following: t max,E = the time at which the C max,E occur at visit E, and t max,C2 = the time at which the C max,C2 occur at visit C2.
The secondary parameters were trough serum concentrations of E3, E2, E1, FSH, LH, and SHBG at all visits; vaginal pH; clinical symptoms (vaginal dryness, vaginal soreness, dyspareunia, and feeling of vaginal discharge) and clinical signs (paleness of the vagina, increased redness of the vaginal walls, ulcerations, and decreased vaginal rugae/mucosal plicae); and the physiological parameters of the vaginal epithelium and microflora, and efficacy, and safety. Vaginal smear samples (wet mount) were taken from the right and left lateral vaginal walls with an Ayre spatula, spread onto two slides, air-dried, and centrally analyzed. The slides were used for immediate pH reading and for microscopic evaluation of the vaginal maturation index (VMI), lactobacillary grade (LBG), bacterial vaginosis (BV) score, AV score, and the presence of Candida. The vaginal pH was measured using Macherey–Nagel pH strips as this provides superior and easy reading [31]. The VMI was calculated based on the percentages of superficial (X3) and intermediate (X2) epithelial cells present in the vaginal smear according to the formula [VMI = 0.5(X2) + 1(X3)]. The vaginal smears for the LBG, BV, and AV score were evaluated in a standardized way, using a phase contrast Leica LM 28 microscope at 400 times magnification as described elsewhere [3, 32, 33]. All slides were anonymized, and randomly read by a person blinded to any clinical information. At each control visit, the global efficacy was assessed by both investigator and patient.
All women receiving at least one dose of study medication were included into the safety analysis set (SAF) and evaluated for adverse events (AEs) or adverse drug reactions (ADRs), and tolerability (by investigator and patient).
Clinical symptoms and medication compliance were recorded in patient’s diary and in sexual questionnaire. Treatment compliance was assessed by asking woman about the medication, checking the medication, and by reviewing the diaries.
The PK variables C max and AUC0–24 were ln-transformed and then compared between day 28 (visit C2) and day 0 (visit E) using a one-way analysis of variance. For concentration-related PK parameters, the geometric mean (GeoMean) was reported and in accordance with the multiplicative model, the coefficient of variation of the geometric mean was calculated as GeoCV = [exp(σ 2) − 1]½, with σ 2 = variance of ln-transformed data. The individual subject values and PK parameters were tabulated with descriptive statistics. Analyses of variance (ANOVA) were performed on AUC0–24 and C max. All PK analyses related to endpoints were performed using a validated software (SAS version 9.2 by SAS Institute, USA). All other variables were analyzed descriptively. Values between visits were compared using the Wilcoxon signed rank sum test, the McNemar test, or the sign test. All continuous parameters were summarized using standard summary statistics as appropriate. All subjects of the SAF set were included in a per-protocol set (PPS) if they completed the study and had no major protocol violations. Adverse events (AEs) were tabulated by MedDRA, and the number and rate of affected subjects were reported. The global assessment of tolerability was reported too.
Results
From 19 screened women, 16 were included in this study, 8 from each center (Fig. 1). One protocol violation was noted: a woman was treated with the steroidal AI exemestane before switched to non-steroidal AI, but was recruited as the investigator was not aware of that. Minor protocol deviations included small shifts from PK measurements schedule, PK sample processing, and shifts in the visits schedule due to inability of patients to attend.
All 16 patients were Caucasian, with a mean age of 57.0 (range 52.0–63.0) years and a body mass index of 23.5 ± 3.0. Patients menstruated a mean of 75.0 (range 25.0–277.0) months ago. The diagnosis of BC had been a median of 2.6 years ago with a range from 2.0 to 28.2 years, and median duration of AI therapy was 2.1 years with a range of 0.5–7.7 years. The daily AI dose was either 1 mg letrozole or 2.5 mg anastrozole. Mean Karnofsky score was 98.1 ± 5.4 %. Mostly used concomitant medications were taken for gastrointestinal tract illnesses (56 %) and for improving the function of the musculoskeletal system, mainly antiphlogistics (44 %).
Treatment compliance was very good during both initial (range 98.7–100.0 %) and maintenance therapy (range 95.8–100.0 %).
Serum E2 and E1 concentrations did not increase at visit E and at visit C2 and were always below the LLOQ, except for one sample of one subject with a E2 concentration just above LLOQ (1.19 pg/ml). After the first application of test medication (visit E, day 0), 15 of 16 women had a transient increase in serum E3 concentration with a C max ranging from 67.6 to 168.0 pg/ml, occurring at 2–3 h post-insertion (Fig. 2). This increase was much lower (maximum 43.70 pg/ml) and occurred later (at 6–8 h) after 4 weeks (visit C2, day 28) and at this visit half of the subjects did not have at all any quantifiable concentrations of E3 (Table 1). The C max (Table 2 ) was significantly lower at visit C2 compared to visit E (p < 0.0001).
E3 levels (Table 3; Fig. 3) were below the LLOQ for all, except two subjects. One had a E3 serum concentration of 22.3 pg/ml at C1, and one had E3 levels of 49.2 pg/ml and 14.5 pg/ml at C1 and C2, respectively. The trough concentrations for E2 and E1 were always below LLOQ.
There were no statistically significant changes of LH and SHBG serum concentrations during treatment. At visit C2, FSH showed a scant, but significant decrease of serum concentration compared to E (p = 0.025), but no significant differences were observed at visits C1, C3, and C4.
VMI improved rapidly already after 2 weeks of treatment from 31 % at entry to 70 % at visit C1 (p < 0.0001) and to 72 % at the end of initial therapy, and was maintained until the end of maintenance therapy at 73 % (Fig. 4, panel a).
Maximum E3 levels inversely correlated with VMI values at visit E and visit C2 (R 2 = 0.62, Fig. 5), demonstrating that the maturing epithelium rapidly precludes further E3 absorption after the initial therapy.
Another important efficacy variable was LBG. At the study entry, the majority of subjects had grossly abnormal vaginal flora (LBG III, 81 %), the remainder being LBG IIb (moderately disturbed). After the 28 days of therapy, almost complete normalisation of the vaginal flora was observed (Fig. 4, panel b): it had become only slightly disturbed (LBGIIa, 63 %) or normal (LBGI, 25 %). Further significant improvements were observed during the maintenance therapy: at C4, the majority of women had a stable and normal vaginal flora (LBGI, 69 %; p = 0.039).
Vaginal pH showed statistically significant decrease (Fig. 4, panel c) from entry (mean 6.0) to visits C1, C2, and C4 (mean 4.4–4.6; p < 0.001), and remained unchanged thereafter during maintenance therapy.
Clinical symptoms of vaginal atrophy like dryness, soreness, and dyspareunia all improved during treatment. Dryness and soreness improved dramatically from entry to control visits (p < 0.001), while statistical evaluation of the improvement in dyspareunia was hampered by low numbers. At entry, sexual intercourse was reported only by 19 % of women, whereas 31 % reported intercourse at visit C4 (p > 0.05). The experience of vaginal discharge increased significantly from entry to C2 (p < 0.01), and then decreased to the end of treatment. Vaginal paleness improved significantly from entry to C2 (p = 0.039) and to C4 (p = 0.006), whereas for redness, ulceration, and rugae, no significant improvement was observed (Table 3).
Almost no women had BV flora at any visit, except at visit C2, where 4 subjects (25 %) presented with partial BV flora (p > 0.05). AV score substantially improved during treatment, from 81 % of patients had moderate to severe AV at entry, to only 37 % with light to moderate AV after initial therapy (p < 0.001). After maintenance therapy, all except one women had returned to normal flora (p < 0.001). The majority of subjects had no Candida colonization at either visit (75–88 %), but at visit C1, 7 women (44 %) were colonized and 4 of them remained colonized during further visits.
From the second week of treatment, the majority of investigators and patients assessed the efficacy as good or very good (>90 and >75 %, respectively) and the evaluations of efficacy by patient significantly increased during maintenance therapy to 94 % at the end of the study period (p = 0.022, Table 3). Global tolerability of the treatment was assessed by both the investigator and patient as good or very good (81–100 %).
No serious AE was reported. All 40 AEs were of mild or moderate severity, of which 15 (62.5 %) were assessed as potentially related to the study medication. The most frequent of them was vaginal discharge.
Discussion
Currently, it is not possible to determine the safety of vaginal estrogen treatment on the basis of data from clinical studies which examine its effect on breast cancer recurrences. Therefore, systemic estrogen absorption and efficacy of intravaginal ultra-low-dose 0.03 mg E3 combined with lactobacilli for vaginal atrophy were tested in a unique study in postmenopausal women on AI therapy. The systemic estrogen levels in NSAI users are much lower as compared to those in healthy postmenopausal women [34]. The use of highly sensitive GC/MS and determining minimal blood level changes of various estrogens are crucial to prove safety in BC patients [35]. In order to clarify this to clinicians taking care of breast cancer patients, we emphasize that routine estrogen assays as they are used in most clinical settings and hospitals are not sufficiently sensitive to guarantee absence of harm due to low levels of circulating estrogens.
In a study, comparing the absorption from a vaginal ring or tablets with E2 in postmenopausal BC patients (no AI therapy), serum levels of E2 and E1 were temporarily raised and efficiently relieved vaginal atrophy [36]. In another study, treatment with low-dose vaginal estrogen (0.25 mg E3 or 12.50 µg E2 twice weekly, for 12 weeks), no increase in E2 or E3 was noted, although no PK values were determined [15]. Vaginal application of a conventional 0.50 mg dose of E3 resulted in increased serum levels of E3, but not E2 and E13 [8–39]. There is fear that even a small, permanent increase in systemic serum estrogen, particularly E1 and E2, may increase the risk of BC recurrence [12, 19]. O’Meara and colleagues reported in their case-controlled study that the risk of recurrence in BC patients who used vaginal estrogens was not increased, irrespective of the total dose and type of estrogen applied [40]. In a study of 69 BC patients suffering from symptoms of vaginal atrophy, vaginal E3 (n = 36) or vaginal E2 (n = 33) was used, with no detrimental effect on recurrence after years of follow-up [41]. A retrospective cohort study in Finland showed that neither the use of vaginal E2 and E3 preparations nor oral E3 were associated with a risk of BC in postmenopausal patients [42]. Hence, strong recommendations either supporting or rejecting the use of various vaginal estrogens in some postmenopausal BC women on AIs are today still difficult to substantiate.
Obviously, not all physiologically available estrogens have the same properties. Whereas E2 and E1 can be reversibly metabolised into each other, E3 is an end product of estrogen metabolism and cannot be transformed back into either E1 or E2 [43, 44]. E3 has been shown to have a 10-times lower affinity to the nuclear ER as compared to E2 [44], and the nuclear retention time of the E3 receptor complex is much shorter (<6 h) than that of the E2 receptor complex (>12 h) [45]. This in combination with its low affinity for plasma proteins and its rapid metabolic clearance turns E3 into a short-acting estrogen [19]. Importantly, it has been consistently reported that vaginal absorption of E3 decreases as the vaginal epithelium matures within a few days to weeks after the start of vaginal treatment [30, 46, 47]. It also has been recognized that in order to exert any stimulatory effect on endometrial and breast tissues, continuous and high doses of E3 use are required [43].
The current study demonstrated that systemic absorption of E3 after administration of Gynoflor® was present, but minimal and transient. After 28 days of vaginal tablet application, most E3 concentrations were below the LLOQ. The exposure (AUC0–24) as well as C max was significantly lower at the end of initial therapy compared with study entry. Trough serum E3, E2, and E1 concentrations remained below their LLOQ for all but 2 patients, where slightly higher levels of E3 were observed. For LH, FSH, and SHBG, only small changes were observed. So, in order to conclude that the application of Gynoflor® in postmenopausal BC patients is oncologically safe, it has to be determined whether this short transient increase of E3 has any influence on BC cells? Although 15 of 16 patients showed this increase after an initial application of Gynoflor, the absorption during therapy decreased (C max decreases and t max increases) as the vaginal epithelium is becoming more mature, as reported in earlier studies [30, 46, 47]. After 4 weeks, daily application of vaginal E3 did not lead to detectable increases in 50 % of patients. The maximum E3 levels were less than 45 pg/ml, and in 25 % of women the highest detected level was below 20 pg/ml. The clinical significance of such transient increases of E3 serum levels is unknown, but probably negligible. In the same period, we did not observe elevations of E2 and E1 levels. In vitro investigations demonstrated that both E2 and E3 can stimulate BC cells, but the effect largely depends on their concentration and duration of action [48]. Hence, the slight and short-lived rise of E3 serum concentrations, without any detectable increase in E2 or E1, can most likely be considered oncologically safe in BC women on AIs. However, even though our observations from this small phase I study are of interest, the ultimate safety of treating women with ER positive breast cancer who are on AIs with small doses of E3 must be demonstrated in larger, properly conducted trials.
Despite the low total absorption of E3, the treatment with ultra-low-dose E3 and lactobacilli combination demonstrated an excellent efficacy: VMI, LBG, vaginal pH normalised, and clinical symptoms of atrophy improved rapidly and dramatically, both during the initial therapy and during the subsequent maintenance therapy. Global efficacy was good or very good, already after 2 weeks of treatment, and improved further till the end of the study. In addition, patients reported an improvement of QoL which is important in BC patients, who frequently report loss of sexual interest and enjoyment after start of anticancer treatment [49]. Gynoflor® was well tolerated. No serious AEs occurred during the study, and none of the AEs were judged as severe.
The strengths of the study were the precise study design, investigation of relevant parameters (PK, safety, and efficacy) in the investigated patient population, and the use of highly sensitive GC/MS to detect minimal changes of systemic estrogen concentrations. The weaknesses of the study were small numbers for testing of some parameters (especially dyspareunia).
In conclusion, 0.03 mg E3 and L. acidophilus vaginal tablets (Gynoflor®) can be considered safe and efficacious for treatment of atrophic vaginitis in BC patients taking AIs. The initial daily vaginal tablet for 4 weeks followed by one application every second day as maintenance therapy led to small and transient increases in serum E3, but not E1 or E2, and therefore seems to be safe in BC patients.
References
Smith IE, Dowsett M (2003) Aromatase inhibitors in breast cancer. N Engl J Med 348(24):2431–2442
Crandall C, Petersen L, Ganz PA, Greendale GA (2004) Association of breast cancer and its therapy with menopause-related symptoms. Menopause 11(5):519–530
Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B (2002) Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG 109(1):34–43
Hickey M, Saunders C, Partridge A, Santoro N, Joffe H, Stearns V (2008) Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol 19(10):1669–1680
Ponzone R, Biglia N, Jacomuzzi ME, Maggiorotto F, Mariani L, Sismondi P (2005) Vaginal oestrogen therapy after breast cancer: is it safe? Eur J Cancer 41(17):2673–2681
Trinkaus M, Chin S, Wolfman W, Simmons C, Clemons M (2008) Should urogenital atrophy in breast cancer survivors be treated with topical estrogens? Oncologist 13(3):222–231
Cardozo L, Benness C, Abbott D (1998) Low dose oestrogen prophylaxis for recurrent urinary tract infections in elderly women. Br J Obstet Gynaecol 105(4):403–407
Suckling J, Lethaby A, Kennedy R (2006) Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 4:CD001500
Bygdeman M, Swahn ML (1996) Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas 23:259–263
Loprinzi CL, Abu-Ghazaleh S, Sloan JA, vanHaelst-Pisani C, Hammer AM, Rowland KM Jr et al (1997) Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol 15(3):969–973
Nachtigall LE (1994) Comparative study: Replens versus local estrogen in menopausal women. Fertil Steril 61(1):178–180
Kendall A, Dowsett M, Folkerd E, Smith I (2006) Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 17(4):584–587
Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J et al (2008) Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 100(7):475–482
Holmberg L, Anderson H (2004) HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet 363(9407):453–455
Biglia N, Peano E, Sgandurra P, Moggio G, Panuccio E, Migliardi M et al (2010) Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: a preliminary study. Gynecol Endocrinol 26:404–412
Labrie F, Cusan L, Gomez JL, Cote I, Berube R, Belanger P et al (2009) Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 16(1):30–36
Notelovitz M, Funk S, Nanavati N, Mazzeo M (2002) Estradiol absorption from vaginal tablets in postmenopausal women. Obstet Gynecol 99(4):556–562
Santen RJ, Pinkerton JV, Conaway M, Ropka M, Wisniewski L, Demers L et al (2002) Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause 9(3):179–187
Al-Baghdadi O, Ewies AA (2009) Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 12(2):91–105
Moegele M, Buchholz S, Seitz S, Ortmann O (2012) Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet 285:1397–1402
Mariani L, Gadducci A, Vizza E, Tomao S, Vici P (2013) Vaginal atrophy in breast cancer survivors: role of vaginal estrogen therapy. Gynecol Endocrinol 29(1):25–29
Del PL (2012) Management of vaginal dryness and dyspareunia in estrogen sensitive cancer patients. Gynecol Endocrinol 28(9):740–745
Chollet JA (2009) Efficacy and safety of vaginal estriol and progesterone in postmenopausal women with atrophic vaginitis. Menopause 16:978–983
Donders GG, Van BB, Van de WP, Kaiser RR, Pohlig G, Gonser S et al (2010) Effect of lyophilized lactobacilli and 0.03 mg estriol (Gynoflor(R)) on vaginitis and vaginosis with disrupted vaginal microflora: a multicenter, randomized, single-blind, active-controlled pilot study. Gynecol Obstet Invest 70(4):264–272
Ozkinay E, Terek MC, Yayci M, Kaiser R, Grob P, Tuncay G (2005) The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 112(2):234–240
Kanne B, Patz B, Wackerle L (1986) Local treatment of vaginal infections with Doederlein bacteria and estriol in climacterium and senium. Frauenarzt 3:35–40
Kanne B (1989) Local administration of weakly dosed estriol and active Lactobacillus acidophilus in the postmenopausal period. Arch Gynecol Obstet 246(Supplement):134
Kanne B, Jenny J (1991) Local administration of low-dosed estriol and viable Lactobacillus acidophilus in the post-menopausal period. Gynäkol Rundsch 31(1):7–13
Jaisamrarn U, Triratanachat S, Chaikittisilpa S, Grob P, Prasauskas V, Taechakraichana N (2013) Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy. Climacteric 16(3):347–355
Kaiser RR, Michael-Hepp J, Weber W, Graf F, Lauritzen C (2000) Absorption of estriol from vaginal tablets after single and repeated application in healthy, postmenopausal women. Therapiewoche 3:2–8
Donders GG, Caeyers T, Tydhof P, Riphagen I, Van den Bosch T, Bellen G (2007) Comparison of two types of dipsticks to measure vaginal pH in clinical practice. Eur J Obstet Gynecol Reprod Biol 134(2):220–224
Donders GG (1999) Microscopy of the bacterial flora on fresh vaginal smears. Infect Dis Obstet Gynecol 7(4):177–179
Donders GG (2007) Definition and classification of abnormal vaginal flora. Best Pract Res Clin Obstet Gynaecol 21(3):355–373
Lonning PE, Geisler J (2008) Aromatase inhibitors: assessment of biochemical efficacy measured by total body aromatase inhibition and tissue estrogen suppression. J Steroid Biochem Mol Biol 108(3–5):196–202
Blair IA (2010) Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids 75(4–5):297–306
Wills S, Ravipati A, Venuturumilli P, Kresge C, Folkerd E, Dowsett M et al (2012) Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract 8(3):144–148
Keller PJ, Riedmann R, Fischer M (1980) Oestrone, oestradiol and oestriol content following intravaginal application of oestriol in the postmenopause. Gynäkol Rundsch 20:77–79
Mattsson L-A, Cullberg G (1983) A clinical evaluation of treatment with estriol vaginal cream versus suppository in postmenopausal women. Acta Obstet Gynecol Scand 62:397–401
van Haaften M, Donker GH, Haspels AA, Thijssen JHH (1989) Oestrogen concentrations in plasma, endometrium, myometrium and vagina of postmenopausal women, and effects of vaginal oestriol (E3) and oestradiol (E2) applications. J Steroid Biochem 33(4A):647–653
O’Meara ES, Rossing MA, Daling JR, Elmore JG, Barlow WE, Weiss NS (2001) Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst 93(10):754–762
Dew JE, Wren BG, Eden JA (2003) A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric 6(1):45–52
Lyytinen H, Pukkala E, Ylikorkala O (2006) Breast cancer risk in postmenopausal women using estrogen-only therapy. Obstet Gynecol 108(6):1354–1360
Head KA (1998) Estriol: safety and efficacy. Altern Med Rev 3(2):101–113
van der Vies J (1982) The pharmacology of oestriol. Maturitas 4:291–299
Anderson JN, Peck EJJ, Clark JH (1975) Estrogen-induced uterine responses and growth: relationship to receptor estrogen binding by uterine nuclei. Endocrinology 96:160–167
Heimer G, Englund D (1984) Estriol: absorption after long-term vaginal treatment and gastrointestinal absorption as influenced by a meal. Acta Obstet Gynecol Scand 63:563–567
Buhling KJ, Eydeler U, Borregaard S, Schlegelmilch R, Suesskind M (2012) Systemic bioavailability of estriol following single and repeated vaginal administration of 0.03 mg estriol containing pessaries. Arzneimittelforschung 62(8):378–383
Lattrich C, Stegerer A, Haring J, Schuler S, Ortmann O, Treeck O (2013) Estrogen receptor beta agonists affect growth and gene expression of human breast cancer cell lines. Steroids 78(2):195–202
Derzko C, Elliott S, Lam W (2007) Management of sexual dysfunction in postmenopausal breast cancer patients taking adjuvant aromatase inhibitor therapy. Curr Oncol 14(Suppl 1):S20–S40
Conflict of interest
Prof Dr Gilbert Donders is a member of Global Advisory Board of Medinova AG, Switzerland. Prof Dr Olaf Ortmann has received consultancy fee from Medinova. Dr Philipp Grob and Dr Valdas Prasauskas are employees of Medinova AG, Switzerland.
Disclosure
This study and online free access of the publication was sponsored by Medinova AG, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Donders, G., Neven, P., Moegele, M. et al. Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor®) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat 145, 371–379 (2014). https://doi.org/10.1007/s10549-014-2930-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2930-x