Abstract
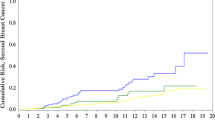
Germline mutations in BRCA1/2 confer a high risk of breast cancer (BC), but the magnitude of this risk varies according to various factors. Although controversial, there are data to support the hypothesis of allelic-risk heterogeneity. We assessed variation in BC risk according to the location of mutations recorded in the French study GENEPSO. Since the women in this study were selected from high-risk families, oversampling of affected women was eliminated by using a weighted Cox-regression model. Women were censored at the date of diagnosis when affected by any cancer, or the date of interview when unaffected. A total of 990 women were selected for the analysis: 379 were classified as affected, 611 as unaffected. For BRCA1, there was some evidence of a central region where the risk of BC is lower (codons 374–1161) (HR = 0.59, P = 0.04). For BRCA2, there was a strong evidence for a region at decreased risk (codons 957–1827) (HR = 0.35, P = 0.005) and for one at increased risk (codons 2546–2968) (HR = 3.56, P = 0.01). Moreover, we found an important association between radiation exposure from chest X-rays and BC risk (HR = 4.29, P < 10−3) and a positive association between smoking more than 21 pack-years and BC risk (HR = 2.09, P = 0.04). No significant variation in BC risk associated with chest X-ray exposure, smoking, and alcohol consumption was found according to the location of the mutation in BRCA1 and BRCA2. Our findings are consistent with those suggesting that the risk of BC is lower in the central regions of BRCA1/2. A new high-risk region in BRCA2 is described. Taking into account environmental and lifestyle modifiers, the location of mutations might be important in the clinical management of BRCA mutation carriers.


Similar content being viewed by others
References
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72:1117–1130
McGuire V, John EM, Felberg A, Haile RW, Boyd NF, Thomas DC, Jenkins MA, Milne RL, Daly MB, Ward J, Terry MB, Andrulis IL, Knight JA, Godwin AK, Giles GG, Southey M, West DW, Hopper JL, Whittemore AS (2006) No increased risk of breast cancer associated with alcohol consumption among carriers of BRCA1 and BRCA2 mutations ages <50 years. Cancer Epidemiol Biomarkers Prev 15:1565–1567
Moorman PG, Iversen ES, Marcom PK, Marks JR, Wang F, Lee E, Ursin G, Rebbeck TR, Domchek SM, Arun B, Susswein L, Isaacs C, Garber JE, Visvanathan K, Griffin CA, Sutphen R, Brzosowicz J, Gruber S, Finkelstein DM, Schildkraut JM (2010) Evaluation of established breast cancer risk factors as modifiers of BRCA1 or BRCA2: a multi-center case-only analysis. Breast Cancer Res Treat 124:441–451
Ghadirian P, Lubinski J, Lynch H, Neuhausen SL, Weber B, Isaacs C, Baruch RG, Randall S, Ainsworth P, Friedman E, Horsman D, Tonin P, Foulkes WD, Tung N, Sun P, Narod SA (2004) Smoking and the risk of breast cancer among carriers of BRCA mutations. Int J Cancer 110:413–416
Ginsburg O, Ghadirian P, Lubinski J, Cybulski C, Lynch H, Neuhausen S, Kim-Sing C, Robson M, Domchek S, Isaacs C, Klijn J, Armel S, Foulkes WD, Tung N, Moller P, Sun P, Narod SA (2009) Smoking and the risk of breast cancer in BRCA1 and BRCA2 carriers: an update. Breast Cancer Res Treat 114:127–135
Nkondjock A, Robidoux A, Paredes Y, Narod SA, Ghadirian P (2006) Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res Treat 98:285–294
Gronwald J, Byrski T, Huzarski T, Cybulski C, Sun P, Tulman A, Narod SA, Lubinski J (2006) Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat 95:105–109
Brunet JS, Ghadirian P, Rebbeck TR, Lerman C, Garber JE, Tonin PN, Abrahamson J, Foulkes WD, Daly M, Wagner-Costalas J, Godwin A, Olopade OI, Moslehi R, Liede A, Futreal PA, Weber BL, Lenoir GM, Lynch HT, Narod SA (1998) Effect of smoking on breast cancer in carriers of mutant BRCA1 or BRCA2 genes. J Natl Cancer Inst 90:761–766
Colilla S, Kantoff PW, Neuhausen SL, Godwin AK, Daly MB, Narod SA, Garber JE, Lynch HT, Brown M, Weber BL, Rebbeck TR (2006) The joint effect of smoking and AIB1 on breast cancer risk in BRCA1 mutation carriers. Carcinogenesis 27:599–605
Breast Cancer Family Registry; Kathleen Cunningham Consortium for Research into Familial Breast Cancer (Australasia); Ontario Cancer Genetics Network (Canada) (2008) Smoking and risk of breast cancer in carriers of mutations in BRCA1 or BRCA2 aged less than 50 years. Breast Cancer Res Treat 109:67–75
Gronwald J, Pijpe A, Byrski T, Huzarski T, Stawicka M, Cybulski C, van Leeuwen F, Lubinski J, Narod SA (2008) Early radiation exposures and BRCA1-associated breast cancer in young women from Poland. Breast Cancer Res Treat 112:581–584
Andrieu N, Easton DF, Chang-Claude J, Rookus MA, Brohet R, Cardis E, Antoniou AC, Wagner T, Simard J, Evans G, Peock S, Fricker JP, Nogues C, Van’t Veer L, van Leeuwen FE, Goldgar DE (2006) Effect of chest X-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group. J Clin Oncol 24:3361–3366
Goldfrank D, Chuai S, Bernstein JL, Ramon YC, Lee JB, Alonso MC, Diez O, Baiget M, Kauff ND, Offit K, Robson M (2006) Effect of mammography on breast cancer risk in women with mutations in BRCA1 or BRCA2. Cancer Epidemiol Biomarkers Prev 15:2311–2313
Narod SA, Lubinski J, Ghadirian P, Lynch HT, Moller P, Foulkes WD, Rosen B, Kim-Sing C, Isaacs C, Domchek S, Sun P (2006) Screening mammography and risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet Oncol 7:402–406
Millikan RC, Player JS, Decotret AR, Tse CK, Keku T (2005) Polymorphisms in DNA repair genes, medical exposure to ionizing radiation, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 14:2326–2334
Gayther SA, Mangion J, Russell P, Seal S, Barfoot R, Ponder BA, Stratton MR, Easton D (1997) Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet 15:103–105
Thompson D, Easton D (2001) Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 68:410–419
Thompson D, Easton D (2002) Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiol Biomarkers Prev 11:329–336
Lubinski J, Phelan CM, Ghadirian P, Lynch HT, Garber J, Weber B, Tung N, Horsman D, Isaacs C, Monteiro AN, Sun P, Narod SA (2004) Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer 3:1–10
Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, Jack E, Vesprini DJ, Kuperstein G, Abrahamson JL, Fan I, Wong B, Narod SA (2001) Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 68:700–710
Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, Narod SA (2006) Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst 98:1694–1706
Evans DG, Shenton A, Woodward E, Lalloo F, Howell A, Maher ER (2008) Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 8:155
Begg CB, Haile RW, Borg A, Malone KE, Concannon P, Thomas DC, Langholz B, Bernstein L, Olsen JH, Lynch CF, Anton-Culver H, Capanu M, Liang X, Hummer AJ, Sima C, Bernstein JL (2008) Variation of breast cancer risk among BRCA1/2 carriers. JAMA 299:194–201
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G (2002) Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30:e57
Casilli F, Di Rocco ZC, Gad S, Tournier I, Stoppa-Lyonnet D, Frebourg T, Tosi M (2002) Rapid detection of novel BRCA1 rearrangements in high-risk breast-ovarian cancer families using multiplex PCR of short fluorescent fragments. Hum Mutat 20:218–226
Barrois M, Bieche I, Mazoyer S, Champeme MH, Bressac-de Paillerets B, Lidereau R (2004) Real-time PCR-based gene dosage assay for detecting BRCA1 rearrangements in breast-ovarian cancer families. Clin Genet 65:131–136
Rouleau E, Lefol C, Bourdon V, Coulet F, Noguchi T, Soubrier F, Bieche I, Olschwang S, Sobol H, Lidereau R (2009) Quantitative PCR high-resolution melting (qPCR-HRM) curve analysis, a new approach to simultaneously screen point mutations and large rearrangements: application to MLH1 germline mutations in Lynch syndrome. Hum Mutat 30:867–875
Weber J, Miserere S, Champ J, Looten R, Stoppa-Lyonnet D, Viovy JL, Houdayer C (2007) High-throughput simultaneous detection of point mutations and large-scale rearrangements by CE. Electrophoresis 28:4282–4288
Gad S, Aurias A, Puget N, Mairal A, Schurra C, Montagna M, Pages S, Caux V, Mazoyer S, Bensimon A, Stoppa-Lyonnet D (2001) Color bar coding the BRCA1 gene on combed DNA: a useful strategy for detecting large gene rearrangements. Genes Chromosomes Cancer 31:75–84
Rouleau E, Lefol C, Tozlu S, Andrieu C, Guy C, Copigny F, Nogues C, Bieche I, Lidereau R (2007) High-resolution oligonucleotide array-CGH applied to the detection and characterization of large rearrangements in the hereditary breast cancer gene BRCA1. Clin Genet 72:199–207
Antoniou AC, Goldgar DE, Andrieu N, Chang-Claude J, Brohet R, Rookus MA, Easton DF (2005) A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol 29:1–11
Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, Falcini F, Franceschi S (2008) Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer 123:2188–2194
Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M (2010) Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev 19:366–373
Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA (2007) Smoking and survival after breast cancer diagnosis. Int J Cancer 120:2672–2677
Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, Caan BJ (2010) Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol 28:4410–4416
Feigelson HS, Calle EE, Robertson AS, Wingo PA, Thun MJ (2001) Alcohol consumption increases the risk of fatal breast cancer (United States). Cancer Causes Control 12:895–902
Marston NJ, Richards WJ, Hughes D, Bertwistle D, Marshall CJ, Ashworth A (1999) Interaction between the product of the breast cancer susceptibility gene BRCA2 and DSS1, a protein functionally conserved from yeast to mammals. Mol Cell Biol 19:4633–4642
Li J, Zou C, Bai Y, Wazer DE, Band V, Gao Q (2006) DSS1 is required for the stability of BRCA2. Oncogene 25:1186–1194
Ma H, Hill CK, Bernstein L, Ursin G (2008) Low-dose medical radiation exposure and breast cancer risk in women under age 50 years overall and by estrogen and progesterone receptor status: results from a case-control and a case–case comparison. Breast Cancer Res Treat 109:77–90
Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW Jr, Coates RJ, Liff JM, Talamini R, Chantarakul N, Koetsawang S, Rachawat D, Morabia A, Schuman L, Stewart W, Szklo M, Bain C, Schofield F, Siskind V, Band P, Coldman AJ, Gallagher RP, Hislop TG, Yang P, Kolonel LM, Nomura AM, Hu J, Johnson KC, Mao Y, De Sanjose S, Lee N, Marchbanks P, Ory HW, Peterson HB, Wilson HG, Wingo PA, Ebeling K, Kunde D, Nishan P, Hopper JL, Colditz G, Gajalanski V, Martin N, Pardthaisong T, Silpisornkosol S, Theetranont C, Boosiri B, Chutivongse S, Jimakorn P, Virutamasen P, Wongsrichanalai C, Ewertz M, Adami HO, Bergkvist L, Magnusson C, Persson I, Chang-Claude J, Paul C, Skegg DC, Spears GF, Boyle P, Evstifeeva T, Daling JR, Hutchinson WB, Malone K, Noonan EA, Stanford JL, Thomas DB, Weiss NS, White E, Andrieu N, Bremond A, Clavel F, Gairard B, Lansac J, Piana L, Renaud R, Izquierdo A, Viladiu P, Cuevas HR, Ontiveros P, Palet A, Salazar SB, Aristizabel N, Cuadros A, Tryggvadottir L, Tulinius H, Bachelot A, Le MG, Peto J, Franceschi S, Lubin F, Modan B, Ron E, Wax Y, Friedman GD, Hiatt RA, Levi F, Bishop T, Kosmelj K, Primic-Zakelj M, Ravnihar B, Stare J, Beeson WL, Fraser G, Bullbrook RD, Cuzick J, Duffy SW, Fentiman IS, Hayward JL, Wang DY, McMichael AJ, McPherson K, Hanson RL, Leske MC, Mahoney MC, Nasca PC, Varma AO, Weinstein AL, Moller TR, Olsson H, Ranstam J, Goldbohm RA, van den Brandt PA, Apelo RA, Baens J, de la Cruz JR, Javier B, Lacaya LB, Ngelangel CA, La Vecchia C, Negri E, Marubini E, Ferraroni M, Gerber M, Richardson S, Segala C, Gatei D, Kenya P, Kungu A, Mati JG, Brinton LA, Hoover R, Schairer C, Spirtas R, Lee HP, Rookus MA, van Leeuwen FE, Schoenberg JA, McCredie M, Gammon MD, Clarke EA, Jones L, Neil A, Vessey M, Yeates D, Appleby P, Banks E, Beral V, Bull D, Crossley B, Goodill A, Green J, Hermon C, Key T, Langston N, Lewis C, Reeves G, Collins R, Doll R, Peto R, Mabuchi K, Preston D, Hannaford P, Kay C, Rosero-Bixby L, Gao YT, Jin F, Yuan JM, Wei HY, Yun T, Zhiheng C, Berry G, Cooper BJ, Jelihovsky T, MacLennan R, Shearman R, Wang QS, Baines CJ, Miller AB, Wall C, Lund E, Stalsberg H, Shu XO, Zheng W, Katsouyanni K, Trichopoulou A, Trichopoulos D, Dabancens A, Martinez L, Molina R, Salas O, Alexander FE, Anderson K, Folsom AR, Hulka BS, Bernstein L, Enger S, Haile RW, Paganini-Hill A, Pike MC, Ross RK, Ursin G, Yu MC, Longnecker MP, Newcomb P, Bergkvist L, Kalache A, Farley TM, Holck S, Meirik O (2002) Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58, 515 women with breast cancer and 95, 067 women without the disease. Br J Cancer 87:1234–1245
Acknowledgments
This GENEPSO study is supported by the Fondation de France and the Ligue Nationale Contre le Cancer. The authors thank Marie-Lise Manche-Thévenot, Claude Picard, and Irwin Piot (hôpital René Huguenin, Saint Cloud, France) who provided technical assistance.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
GENEPSO Collaborating Centers are listed in Appendix.
Appendix
Appendix
GENEPSO Collaborating Centers
Coordinating Center, Hôpital René Huguenin/Institut Curie,Saint Cloud: Catherine Noguès, Emmanuelle Fourme, Rosette Lidereau; Etienne Rouleau, Sandrine Caputo.
Collaborating Centers: Institut Curie, Paris: Dominique Stoppa-Lyonnet, Marion Gauthier-Villars; Bruno Buecher, Institut Gustave Roussy, Villejuif: Olivier Caron; Hôpital René Huguenin/Institut Curie, Saint Cloud: Catherine Noguès, Liliane Demange; Centre Paul Strauss, Strasbourg: Jean-Pierre Fricker; Centre Léon Bérard, Lyon: Christine Lasset, Valérie Bonadona; Centre François Baclesse, Caen: Pascaline Berthet; Hôpital d’Enfants CHU Dijon – Centre Georges François Leclerc, Dijon: Laurence Faivre; Centre Alexis Vautrin, Vandoeuvre-les-Nancy: Elisabeth Luporsi; Centre Antoine Lacassagne, Nice: Marc Frénay; Institut Claudius Regaud, Toulouse: Laurence Gladieff; Réseau Oncogénétique Poitou Charente, Niort: Paul Gesta; Institut Paoli-Calmettes, Marseille: Hagay Sobol, François Eisinger, Laetitia Huiart; Institut Bergonié, Bordeaux: Michel Longy; Centre Eugène Marquis, Rennes: Catherine Dugast; GH Pitié Salpétrière, Paris: Chrystelle Colas, Florent Soubrier; CHU Arnaud de Villeneuve, Montpellier: Isabelle Coupier, Pascal Pujol; Centres Paul Papin, and Catherine de Sienne, Angers, Nantes: Alain Lortholary; Centre Oscar Lambret, Lille: Philippe Vennin, Claude Adenis; Institut Jean Godinot, Reims: Tan Dat Nguyen; Centre René Gauducheau, Nantes: Capucine Delnatte; Centre Henri Becquerel, Rouen: Annick Rossi, Julie Tinat, Isabelle Tennevet; Hôpital Civil, Strasbourg: Jean-Marc Limacher, Christine Maugard; Hôpital Centre Jean Perrin, Clermont-Ferrand: Yves-Jean Bignon; Polyclinique Courlancy, Reims: Liliane Demange; Clinique Sainte Catherine, Avignon: Hélène Dreyfus; Hôpital Saint-Louis, Paris: Odile Cohen-Haguenauer; CHRU Dupuytren, Limoges: Brigitte Gilbert; Couple-Enfant-CHU de Grenoble: Dominique Leroux; Hôpital de la Timone, Marseille: Hélène Zattara-Cannoni; Inserm U900, Ecole des Mines de Paris, ParisTech, Service de Biostatistiques, Institut Curie, Paris: Nadine Andrieu; Inserm U535, Villejuif: Catherine Bonaïti; Inserm U379, Marseille: Claire Julian-Reynier.
Rights and permissions
About this article
Cite this article
Lecarpentier, J., Noguès, C., Mouret-Fourme, E. et al. Variation in breast cancer risk with mutation position, smoking, alcohol, and chest X-ray history, in the French National BRCA1/2 carrier cohort (GENEPSO). Breast Cancer Res Treat 130, 927–938 (2011). https://doi.org/10.1007/s10549-011-1655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1655-3