Abstract
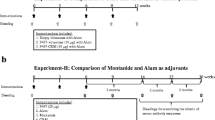
We have previously shown in mice that vaccination with three Her-2-peptides representing B-cell epitopes of the extracellular domain of Her-2/neu induces Her-2/neu-specific IgG antibodies with strong anti-tumor activity in vitro and in vivo. We have now finalized a phase I clinical trial with an anti-Her-2/neu vaccine-construct of immunopotentiating reconstituted influenza virosomes with the three peptides in patients with metastatic breast cancer (MBC). Ten MBC patients with low protein overexpression of Her-2/neu of MBC (+ or ++ upon immunohistochemistry, FISH negative) and positive hormone receptor status were enrolled in a single center phase I study. The virosomal formulated vaccine, consisting of 10 μg/peptide, was intramuscularly applied three times on days 1, 28, and 56. The primary endpoint of the study, which lasted 12 weeks, was safety, the secondary endpoint immunogenicity. Local erythema at the injection site was the only vaccine-related side effect occurring in four patients. In 8 of 10 patients an increase in peptide-specific antibody titer measured by ELISA was found. Importantly, the induced antibodies were also directed against the native Her-2/neu protein. Cellular immune responses, as measured by in vitro production of IL-2, IFN-γ, and TNF-α of PBMCs showed a marked increase after vaccination in the majority of vaccinees. Notably, the number of CD4+CD25+Foxp3+T regulatory cells, which were significantly increased compared to healthy controls prior to vaccination, was markedly reduced following vaccination. In all, the immunological responses after vaccination indicated that the patients in stage IV of disease were immunocompetent and susceptible to vaccination. The Her-2/neu multipeptide vaccine was safe, well tolerated and effective in overcoming immunological tolerance to Her-2/neu. The induction of anti-Her-2-specific antibodies could result in clinical benefit comparable to passive anti-Her-2 antibody therapy.



Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Andrulis IL, Bull SB, Blackstein ME et al (1998) neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16(4):1340–1349
Suter TM, Procter M, van Veldhuisen DJ et al (2007) Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 25(25):3859–3865
Maloney DG, Grillo-Lopez AJ, Bodkin DJ et al (1997) IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 15(10):3266–3274
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10(9):909–915
Lollini PL, Cavallo F, Nanni P, Forni G (2006) Vaccines for tumour prevention. Nat Rev Cancer 6(3):204–216
Jasinska J, Wagner S, Radauer C et al (2003) Inhibition of tumor cell growth by antibodies induced after vaccination with peptides derived from the extracellular domain of Her-2/neu. Int J Cancer 107(6):976–983
Wagner S, Jasinska J, Breiteneder H, Kundi M, Pehamberger H, Scheiner O, Zielinski CC, Wiedermann U (2007) Delayed tumor onset and reduced tumor growth progression after immunization with a Her-2/neu multi-peptide vaccine and IL-12 in c-neu transgenic mice. Breast Cancer Res Treat 106(1):29–38
Zurbriggen R (2003) Immunostimulating reconstituted influenza virosomes. Vaccine 21(9–10):921–924
Bovier PA (2008) Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines 7(8):1141–1150
Thompson FM, Porter DW, Okitsu SL et al (2008) Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS ONE 3(1):e1493
Garner-Spitzer E, Kundi M, Rendi-Wagner P et al (2009) Correlation between humoral and cellular immune responses and the expression of the hepatitis A receptor HAVcr-1 on T cells after hepatitis A re-vaccination in high and low-responder vaccinees. Vaccine 27(2):197–204
Phillips CA, Forsyth BR, Christmas WA, Gump DW, Whorton EB, Rogers I, Rudin A (1970) Purified influenza vaccine: clinical and serologic responses to varying doses and different routes of immunization. J Infect Dis 122(1):26–32
Zielinski CC, Stuller I, Dorner F, Potzi P, Muller C, Eibl MM (1986) Impaired primary, but not secondary, immune response in breast cancer patients under adjuvant chemotherapy. Cancer 58(8):1648–1652
Molling JW, Kolgen W, van der Vliet HJ et al (2005) Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer 116(1):87–93
Liyanage UK, Moore TT, Joo HG et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169(5):2756–2761
Fisk B, Blevins TL, Wharton JT, Ioannides CG (1995) Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 181(6):2109–2117
Disis ML, Grabstein KH, Sleath PR, Cheever MA (1999) Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 5(6):1289–1297
Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA (1996) Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol 156(9):3151–3158
Matsui S, Ahlers JD, Vortmeyer AO, Terabe M, Tsukui T, Carbone DP, Liotta LA, Berzofsky JA (1999) A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol 163(1):184–193
Welters MJ, Kenter GG, Piersma SJ et al (2008) Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 14(1):178–187
Peoples GE, Holmes JP, Hueman MT et al (2008) Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res 14(3):797–803
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H (1998) The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188(12):2357–2368
Kunzi V, Dornseiff M, Horwath J, Hartmann K (2009) Safe vaccination of children with a virosomal adjuvanted influenza vaccine. Vaccine 27(8):1261–1265
Coveler AL, Goodell V, Webster DJ, Salazar LG, Fintak PA, Childs JS, Higgins DM, Disis ML (2009) Common adjuvant breast cancer therapies do not inhibit cancer vaccine induced T cell immunity. Breast Cancer Res Treat 113(1):95–100
Disis ML, Goodell V, Schiffman K, Knutson KL (2004) Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol 24(5):571–578
Goldblatt D, Vaz AR, Miller E (1998) Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis 177(4):1112–1115
Pfister G, Weiskopf D, Lazuardi L et al (2006) Naive T cells in the elderly: are they still there? Ann N Y Acad Sci 1067:152–157
Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J (2009) The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer 100(7):1061–1067
Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H (2008) Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer 98(1):148–153
Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H (2006) CD4(+)CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 55(9):1064–1071
Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H (2007) Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci USA 104(52):20884–20889
Enarsson K, Lundgren A, Kindlund B, Hermansson M, Roncador G, Banham AH, Lundin BS, Quiding-Jarbrink M (2006) Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin Immunol 121(3):358–368
Generali D, Bates G, Berruti A et al (2009) Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res 15(3):1046–1051
Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, dela Rosa C, Cheever MA (2004) Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein-based vaccine. J Clin Oncol 22(10):1916–1925
Holmes JP, Gates JD, Benavides LC et al (2008) Optimal dose and schedule of an HER-2/neu (E75) peptide vaccine to prevent breast cancer recurrence: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer 113(7):1666–1675
Acknowledgments
The study was supported by a grant from BioLife Science and by the Medical University of Vienna. The assistance of Mrs Marika Rosner as study nurse during the phase I clinical trial is very much appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
U. Wiedermann and C. Wiltschke contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wiedermann, U., Wiltschke, C., Jasinska, J. et al. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res Treat 119, 673–683 (2010). https://doi.org/10.1007/s10549-009-0666-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0666-9