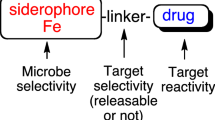
Abstract
The outer membrane permeability barrier is an important resistance factor of bacterial pathogens. In combination with drug inactivating enzymes, target alteration and efflux, it can increase resistance dramatically. A strategy to overcome this membrane-mediated resistance is the misuse of bacterial transport systems. Most promising are those for iron transport. They are vital for virulence and survival of bacteria in the infected host, where iron depletion is a defense mechanism against invading pathogens. We synthesized biomimetic siderophores as shuttle vectors for active transport of antibiotics through the bacterial membrane. Structure activity relationship studies resulted in siderophore aminopenicillin conjugates that were highly active against Gram-negative pathogens which play a crucial role in destructive lung infections in cystic fibrosis patients and in severe nosocomial infections. The mechanism of action and the uptake of the compounds via specific iron siderophore transport routes were demonstrated. The novel conjugates were active against systemic Pseudomonas aeruginosa infections in mice with ED50 values comparable to the quinolone ofloxacin and show low toxicity.




Similar content being viewed by others
References
Ankel-Fuchs D, Matthiesen T, Bauernfeind A, Möllmann U, Heinisch L, Härtl A (2000) In vitro and in vivo characteristics of new siderophore-β-lactam conjugates with enhanced activity against glucose-non-fermenting Gram-negative bacteria. 10th ECCMID, Stockholm, Sweden, May 2000. Abstract WeP171
Arisawa M, Sekine Y, Shimizu S, Takano H, Angehrn P (1991) In vitro and in vivo evaluation of Ro 09–1428, a new parenteral cephalosporin with high antipseudomonal activity. Antimicrob Agents Chemother 35:653–659
Benz G, Schröder T, Kurz J, Wünsche C, Karl W, Steffen GJ, Pfitzner J, Schmidt D (1982) Konstitution der Deferriform der Albomycine δ1, δ2 und ε. Angew Chem 94:552–553
Bickel H, Gäumann E, Nussberg G, Reusser P, Vischer E, Voser W, Wettstein A, Zähner H (1960) Stoffwechselprodukte von Actinomyceten. 25 Mitteilung: Über die isolierung und charakterisierung der ferrimycine A1 und A2, neuer antibiotika der sideromycin-gruppe. Helv Chim Acta 43:2105–2118. doi:10.1002/hlca.19600430730
Bickel H, Mertens P, Prelog V, Seibl J, Walser A (1965) Constitution of ferrimycin A1. Antimicrob Agents Chemother 5:951–957
Braun V, Killmann H (1999) Bacterial solutions to the iron-supply problem. Trends Biochem Sci 24:104–109. doi:10.1016/S0968-0004(99)01359-6
Chang MH, Koh HY, Choh YS, Choi KI (1997) Novel anti-Pseudomonas Cephalosporins. Curr Pharm Des 3:209–226
Fiedler H-P, Walz F, Zähner H (1985) Albomycin: studies on fermentation, isolation, and quantitative determination. Appl Microbiol Biotechnol 21:341–347. doi:10.1007/BF00249977
Gause GF (1955) Recent studies on albomycin, a new antibiotic. BMJ 12:1177–1179
Ghosh A, Ghosh M, Niu C, Malouin F, Moellmann U, Miller MJ (1996) Iron transport-mediated drug delivery using mixed-ligand siderophore-beta-lactam conjugates. Chem Biol 3:1011–1019. doi:10.1016/S1074-5521(96)90167-2
Guerinot ML (1994) Microbial iron transport. Annu Rev Microbiol 48:743–772. doi:10.1146/annurev.mi.48.100194.003523
Guinea J, Cercenado E, Garcia-Garrote F, Cuevas O, Martin-Pedroviejo J, Bouza E (2000) Prevalence of efflux pumps among clinical isolates of multiple antibiotic resistant Pseudomonas aeruginosa. Abstr Intersci Conf Antimicrob Agents Chemother 40:87
Hantke K (1990) Dihydroxybenzoylserine, a siderophore for E.coli. FEMS Microbiol Lett 67:5–8
Heinisch L, Gebhardt P, Heidersbach R, Reissbrodt R, Möllmann U (2002a) New synthetic catecholate-type siderophores with triamine backbone. Biometals 15:133–144. doi:10.1023/A:1015293900133
Heinisch L, Wittmann S, Stoiber T, Berg A, Ankel-Fuchs D, Möllmann U (2002b) Highly antibacterial active aminoacyl penicillin conjugates with bis-catecholate siderophores based on secondary diamino acids and related compound. J Med Chem 45:3032–3040. doi:10.1021/jm010546b
Heinisch L, Wittmann S, Stoiber T, Scherlitz-Hoffmann I, Ankel-Fuchs D, Möllmann U (2003) New tris- and tetrakis-catecholate siderophores based on polyazaalkanoic acids and their β-lactam conjugates. Arzneim-Forschung. Drug Res 53:188–195
Köhler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty LK, Pechère JC (1997) Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol 23:345–354. doi:10.1046/j.1365-2958.1997.2281594.x
Mies KA, Gebhardt P, Möllmann U, Crumbliss AL (2008) Synthesis, siderophore activity and iron(III) chelation chemistry of a novel mono-hydroxamate, bis-catecholate siderophore mimic: N(alpha),-N(epsilon)-bis[2,3-dihydroxybenzoyl]-l-lysyl-(gamma-N-methyl-N-hydroxyamido)-l-glutamic acid. J Inorg Biochem 102:850–861. doi:10.1016/j.jinorgbio.2007.11.021
Möllmann U, Heinisch L, Ankel-Fuchs D, Köhler T (2001a) Mechanism of action of new Siderophore Acylaminopenicillins. Abstr Intersci Conf Antimicrob Agents Chemother 16–19 Dec 2001, 41 abstract no. F-377
Möllmann U, Heinisch L, Bauernfeind A, Schneider I, Schmitz FJ, Ankel-Fuchs D (2001b) Antibacterial activity of new Siderophore Acylaminopenicillins. Abstr Intersci Conf Antimicrob Agents Chemother 16–19 Dec 2001, 41 abstract no. F-378
Neilands JB (1982) Microbial envelope proteins related to iron. Annu Rev Microbiol 36:285–309. doi:10.1146/annurev.mi.36.100182.001441
O’Brien IG, Cox GB, Gibson F (1970) Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim Biophys Acta 201:453–460
O’Brien IG, Cox GB, Gibson F (1971) Enterochelin hydrolysis and iron metabolism in Escherichia coli. Biochim Biophys Acta 237:537–549
Pollack JR, Neilands JB (1970) Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun 38:989–992. doi:10.1016/0006-291X(70)90819-3
Pramanik A, Braun V (2006) Albomycin uptake via a ferric hydroxamate transport system of Streptococcus pneumoniae R6. J Bacteriol 188:3878–3886
Raymond KN, Dertz EA, Sanggoo SK (2003) Bioinorganic chemistry special feature: Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci USA 100:3584–3588. doi:10.1073/pnas.0630018100
Roosenberg JMII, Lin YM, Lu Y, Miller MJ (2000) Studies and syntheses of siderophores, microbial iron chelators, and analogs as potential drug delivery agents. Curr Med Chem 7:159–197
Sackmann W, Preusser P, Neipp L, Kradolfer F, Gross F (1962) Ferrimycin A, a new iron containing antibiotic. Antibiot Chemother 12:34–45
Schnabelrauch M, Wittmann S, Rahn K, Möllmann U, Reissbrodt R, Heinisch L (2000) New synthetic catecholate-type siderophores based on amino acids and dipeptides. Biometals 13:333–348. doi:10.1023/A:1009297610755
Schneider I, Bauernfeind A, Miehle S, Ankel-Fuchs D (1999) Activity of aminopenicillin-siderophore-conjugates against Stenotrophomonas maltophilia. 39th Interscience Conference on antimicrobial agents and chemotherapy, San Francisco, California, September 1999, Abstract 400, p 298
Schumann G, Möllmann U (2001) A screening system for siderophores as potential drug delivery agents in mycobacteria. Antimicrob Agents Chemother 45:1317–1322. doi:10.1128/AAC.45.5.1317-1322.2001
Schweizer HP (2003) Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet Mol Res 2:48–62
Schwynn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. doi:10.1016/0003-2697(87)90612-9
Shlaes DM, Projan SJ, Edwards JE Jr (2004) Antibiotic discovery: state of the state. ASM News 70:275–281
Talbot GH (2008) What is the pipeline for Gram-negative pathogens? Expert Rev Anti Infect Ther 6:39–49. doi:10.1586/14787210.6.1.39
Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld ME, Bartlett JG (2006) Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infections diseases society of America. Clin Infect Dis 42:657–668. doi:10.1086/499819
Vertesy L, Aretz W, Fehlhaber HW, Kogler H (1995) Salmycin A–D, antibiotika aus Streptomyces violaceus, DSM 8286, mit siderophor-aminoglycosid-struktur (salmycin A–D, antibiotics from Streptomyces violaceus, DSM 8286, having a siderophor-aminoglycoside structure). Helv Chim Acta 78:46–60. doi:10.1002/hlca.19950780105
Weinberg ED (1995) Acquisition of iron and other nutrients in vivo. In: Roth JA et al (eds) Virulence mechanisms of bacterial pathogens. ASM Press, Washington, DC, pp 79–93
Wittmann S, Scherlitz-Hofmann I, Möllmann U, Ankel-Fuchs D, Heinisch L (2000) 8-Acyloxy-1,3-benzoxazine-2,4-diones as siderophore components of antibiotics. Arzneim-Forsch Drug Res 50:752–757
Wittmann S, Schnabelrauch M, Scherlitz-Hoffmann I, Möllmann U, Ankel-Fuchs D, Heinisch L (2002) New synthetic siderophores and their β-lactam conjugates based on amino acids and dipeptides. Bioorg Med Chem 10:1659–1670. doi:10.1016/S0968-0896(02)00044-5
Wright AC, Simpson LM, Oliver JD (1981) Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun 34:503–507
Zähner H, Diddens H, Keller-Schierlein W, Nägeli H-U (1977) Some experiments with semisynthetic sideromycins. Jpn J Antibiot 30:S201–S206
Zimmermann W (1980) Penetration of β-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother 18:94–100
Acknowledgments
We thank Klaus Hantke, University of Tübingen, Germany, for the generous support with the porin mutants as well as with the iron transport mutants, Franz-Josef Schmitz, University of Düsseldorf, Germany, for testing in vitro activity against clinical P. aeruginosa isolates, Monika Golembiewski and Irmgard Heinemann for their excellent technical assistance. Financial support by German Ministry of Education and Research, BMBF grant #0311232 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Möllmann, U., Heinisch, L., Bauernfeind, A. et al. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals 22, 615–624 (2009). https://doi.org/10.1007/s10534-009-9219-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9219-2