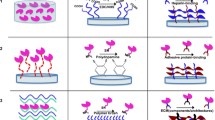
Abstract
The beautifully orchestrated complexity of the temporal spatial growth factor gradients during embryogenesis offer a striking contrast to systemic bolus administration that lack tissue specificity and sustained protein localization, often requiring supraphysiological protein doses to produce the desired therapeutic dose. These attributes may be responsible for clinically observed dangerous tissue overgrowth, inflammation, and even tumor formation. Growth factor delivery within an implanted scaffold is a very attractive way to modulate cell behavior. For short term delivery, proteins can be non-specifically adsorbed to the material surface or simply entrapped within the bulk scaffold. For more sustained delivery, many researchers have turned to the ever increasing list of covalent immobilization methods that have profound applications in purification, biosensing, imaging, and drug discovery by tethering proteins, nucleic acids, carbohydrates, synthetic polymers, small molecules, nanotubes, and even whole cells. This review focuses on the use of covalent immobilization to achieve sustained growth factor delivery for tissue engineering. Covalent immobilization techniques will be reviewed in terms of design, protein bioactivity/stability, efficiency, and spatiotemporal distribution. Further, the biological response to sustained growth factor delivery will also be covered, such as cell interaction, cell responsiveness, proliferation, differentiation, extracellular matrix production, and tissue regeneration. This focused review is anticipated to inform investigators on the selection of optimal immobilization strategies for their specific applications.
Similar content being viewed by others
References
Alsberg, E., D. J. Mooney, et al. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 80(11):2025–2029, 2001.
Anderson, S. M., T. Segura, et al. The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF. Biomaterials 30:4618–4628, 2009.
Bhakta, G., B. Rai, S. M. Cool, et al. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 33:6113–6122, 2012.
Bieniarz, C., M. Husain, et al. Extended length heterobifunctional coupling agents for protein conjugations. Bioconjugate Chem. 7:88–95, 1996.
Binder, W. H., and R. Sachsenhofer. ‘Click’ chemistry in polymer and materials science. Macromol. Rapid Commun. 28:15–54, 2007.
Biondi, M., A. Netti, et al. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 60:229–242, 2008.
Breinan, H. A., T. Minas, M. Spector, et al. Histological evaluation of the course of healing of canine articular cartilage defects treated with cultured autologous chondrocytes. Tissue Eng. 4(1):101–113, 1998.
Budiraharjo, R., K. G. Neoh, and E. Kang. Enhancing bioactivity of chitosan film for osteogenesis and wound healing by covalent immobilization of BMP-2 or FGF-2. J. Biomater. Sci. Polym. Ed. 24(6):645–662, 2013.
Carlsson, J., H. Lundqvist, et al. Conjugate chemistry and cellular processing of EGF-dextran. Acta Oncol. 38(3):313–321, 1999.
Chen, G., and Y. Ito. Gradient micropattern immobilization of EGF to investigate the effect of artificial juxtacrine stimulation. Biomaterials 22:2453–2457, 2001.
Chen, F. M., Z. F. Wu, et al. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 31:6279–6308, 2010.
Chevalier, J., J. Yi, et al. Biotin and digoxigenin as labels for light and electron microscopy in situ hybridization probes: where do we stand? J. Histochem. Cytochem. 45:481, 1997.
DeLong, S. A., A. S. Gobin, and J. L. West. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J. Controlled Release 109:139–148, 2005.
DeLong, S. A., J. J. Moon, and J. L. West. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 26:3227–3234, 2005.
Duan, B., and M. Wang. Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor. J. R. Soc. Interface 7:S615–S629, 2010.
Elia, R., R. A. Peattie, et al. Stimulation of in vivo angiogenesis by an in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials 31:4630–4638, 2010.
Gurdon, J. B., and P. Y. Bourillot. Moprhogen gradient interpretation. Nature 413:797–803, 2001.
Gurdon, J. B., H. Standley, et al. Single cells can sense their position in a morphogen gradient. Development 126:5309–5317, 1999.
Harris, B. P., A. T. Metters, et al. Photopatterned polymer brushes promoting cell adhesion gradients. Langmuir 22(10):4467–4471, 2006.
Ho, Y., F. Mi, H. Sung, and P. Kuo. Heparin-functionalized chitosan-alginate scaffolds for controlled release of growth factor. Intl. J. Pharm. 376:69–75, 2009.
Hubbard, T. J., and C. A. Hicks-Little. Ankle ligament healing after an acute ankle sprain: an evidence-based approach. J. Athletic Training 43(5):523–529, 2008.
Israelachvili, J. Intermolecular and Surface Forces (2nd ed.). London: Academic Press, 1992.
Jabbari, E. Bioconjugation of hydrogels for tissue engineering. Curr. Opin. Biotechnol. 22:655–660, 2011.
Jeon, O., B. S. Kim, et al. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials 28(17):2763–2771, 2007.
Jiskoot, W., T. W. Randolph, et al. Protein instability and immogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J. Pharm. Sci. 101(3):946–954, 2012.
Kapur, T. A., and M. S. Shoichet. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J. Biomed. Mater. Res. 68A:235–243, 2004.
Karageorgiou, V., D. Kaplan, et al. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. A 71A:528–537, 2004.
Lam, H. J., S. Li, et al. In vitro regulation of neural differentiation and axon growth by growth factors and bioactive nanofibers. Tissue Eng. Part A 16(8):2641–2648, 2010.
Langer, R., and M. Moses. Biocompatible controlled release polymers for delivery of polypeptides and growth factors. J. Cell. Biochem. 45:340–345, 1991.
Lee, J., J. J. Yoo, A. Atala, and S. J. Lee. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials 33:6709–6720, 2012.
Leipzig, N. D., M. S. Shoichet, et al. Functional immobilization of interferon-gamma induces neuronal differentiation of neural stem cells. J. Biomed. Mater. Res. 93A:625–633, 2010.
Leipzig, N. C., M. S. Shoichet, et al. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials 32:57–64, 2011.
Liu, H. W., G. H. Hsiue, et al. Heterobifunctional poly(ethylene glycol)-tethered bone morphogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng. 13(5):1113–1124, 2007.
Liu, L. S., R. C. Spiro, et al. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2). J. Biomed. Mater. Res. 62:128–135, 2002.
Liu, H., and T. J. Webster. Ceramic/polymer nanocomposites with tunable drug delivery capability at specific disease sites. J. Biomed. Mater. Res. 93A:1180–1192, 2010.
Lock, J., T. Y. Nguyen, and H. Liu. Nanophase hydroxyapatite and poly(lactide-co-glycolide) composites promote human mesenchymal stem cell adhesion and osteogenic differentiation in vitro. J. Mater. Sci. Mater. Med. 23:2543–2552, 2012.
Lutz, J., and H. G. Borner. Modern trends in polymer bioconjugates design. Prog. Polym. Sci. 33:1–39, 2008.
Mann, B. K., R. H. Schmedlen, and J. L. West. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 22:439–444, 2001.
Marcantonio, N. A., L. G. Griffith, et al. The influence of tethered epidermal growth factor on connective tissue progenitor colony formation. Biomaterials 30:4629–4638, 2009.
Marden, L. J., and J. O. Hollinger. Platelet-derived growth factor inhibits bone regeneration induced by osteogenin, a bone morphogenetic protein, in rat craniotomy defects. J. Clin. Invest. 92:2897–2905, 1993.
Metzger, W., M. Oberringer, et al. Induction of myofibroblastic differentiation in vitro by covalently immobilized transforming growth factor-B1. Tissue Eng. 13(11):2751–2760, 2007.
Molineux, G. Pegylation: engineering improved pharmaceuticals for enhanced therapy. Cancer Treat. Rev. 28(Supplement 1):13–16, 2002.
Moon, J. J., S. H. Lee, and J. L. West. Synthetic biomimetic hydrogels incorporated with ephrin-A1 for therapeutic angiogenesis. Biomacromolecules 8:42–49, 2007.
Moore, K., M. S. Shoichet, et al. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 12:267–278, 2006.
Nillesen, S. T., T. H. van Kuppevelt, et al. Increased angiogenesis and blood vessel maturation in acellular collagen heparin scaffolds containing both FGF2 and VEGF. Biomaterials 28:1123–1131, 2007.
Park, J. S., K. H. Park, et al. Determination of dual delivery for stem cell differentiation using dexamethasone and TGF-beta3 in/on polymeric microspheres. Biomaterials 30:4796–4805, 2009.
Patel, S., S. Li, et al. Regulation of endothelial cell function by GRDGSP peptide grafted onto interpenetrating polymers. J. Biomed. Mater. Res. 83A:423–433, 2007.
Patel, S., S. Li, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 7(7):2122–2128, 2007.
Patel, N., K. M. Shakesheff, et al. Spatially controlled cell engineering on biodegradable polymer surfaces. FASEB J. 12:1447–1454, 1998.
Phelps, E. A., A. J. Garcia, et al. Bioartificial matrices for therapeutic vascularization. PNAS 107(8):3323–3328, 2010.
Pompe, T., C. Werner, et al. Immobilization of growth factors on solid support for the modulation of stem cell fate. Nat. Protoc. 5(6):1042–1050, 2010.
Puccinelli, T. J., P. J. Bertics, and K. S. Masters. Regulation of keratinocyte signaling and function via changes in epidermal growth factor presentation. Acta Biomater. 6(9):3415–3425, 2010.
Quaglia, F. Bioinspired tissue engineering: the great promise of protein delivery technologies. Intl. J. Pharm. 364:281–297, 2008.
Raiche, A. T., and D. A. Puleo. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials 25:677–685, 2004.
Richardson, T. P., D. J. Mooney, et al. Polymeric system for dual growth factory delivery. Nat. Biotechnol. 19:1029–1034, 2001.
Ripamonti, U., D. C. Rueger, et al. Periodontal tissue regeneration by combined applications of recombinant human osteogenic protein-1 and bone morphogenetic protein-2. A pilot study in Chacma baboons (Papio ursinus). Eur. J. Oral Sci. 109:241–248, 2001.
Rohman, G., N. R. Cameron, et al. Heparin functionalization of porous PLGA scaffolds for controlled, biologically relevant delivery of growth factors for soft tissue engineering. J. Mater. Chem. 12:9265–9273, 2009.
Rowley, J. A., and D. J. Mooney. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 60:217–223, 2002.
Schaffer, D. V., and D. A. Lauffenburger. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J. Biol. Chem. 273:28004–28009, 1998.
Sheardown, H., C. Wedge, et al. Continuous epidermal growth factor delivery in corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 34(13):3593–3600, 1993.
Stroncek, J. D., and W. M. Reichert. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Boca Raton: CRC Press, 2008, Chap. 1.
Takita, H., E. Tsuruga, et al. Enhancement by bFGF of osteogenesis induced by rhBMP-2 in rats. Eur. J. Oral Sci. 105:588–592, 1997.
Tan, H., C. Gao, et al. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 18:1961–1968, 2007.
Tayalia, P., and D. J. Mooney. Controlled growth factory delivery for tissue engineering. Adv. Mater. 21:3269–3285, 2009.
Teixeira, S., L. Yang, F. J. Monteiro, et al. Heparinized hydroxyapatite/collagen three-dimensional scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 21:2385–2392, 2010.
Tigli, R. S., and M. Gumusderelioglu. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Intl. J. Biol. Macromol. 43:121–128, 2008.
Van Den Beucken, J. J. J. P., J. A. Jansen, et al. In vitro and in vivo effects of deoxyribonucleic acid-based coatings functionalized with vascular endothelial growth factor. Tissue Eng. 13(4):711–720, 2007.
Vonau, R. L., and A. E. Sams, et al. Combination of growth factors inhibits bone ingrowth in the bone harvest chamber. Clin. Orthop. 386:243–251, 2001.
Wissink, M. J. B., J. Feijen, et al. Binding and release of basic fibroblast growth factor from heparinized collagen matrices. Biomaterials 22(16):2291–2299, 2001.
Yoon, J. J., T. G. Park, et al. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J. Biomed. Mater. Res. Part A 79A(4):934–942, 2006.
Yoon, J. J., T. G. Park, et al. Photo-crosslinkable and biodegradable Pluronic/heparin hydrogels for local delivery of angiogenic growth factor. J. Biomed. Mater. Res. A 83A(3):597–605, 2007.
Young, S., L. S. Baggett, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng. Part A 15:2347–2362, 2009.
Yu, J., S. Li, et al. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials 33:8062–8074, 2012.
Zhang, H., S. J. Hollister, et al. Chemically-conjugated bone morphogenetic protein-2 on three-dimensional polycaprolactone (PCL) scaffolds stimulates osteogenic activity in bone marrow stromal cells. Tissue Eng.: Part A 16(11):3441–3448, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Tzung Hsiai oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Reed, S., Wu, B. Sustained Growth Factor Delivery in Tissue Engineering Applications. Ann Biomed Eng 42, 1528–1536 (2014). https://doi.org/10.1007/s10439-013-0956-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0956-6