Abstract
Background
Programmed cell death ligand 1 (PD-L1) regulates immune responses through interaction with its receptor. PD-L1 is not only a predictor of poor prognosis but also a new therapeutic target in several malignancies. Neoadjuvant chemoradiotherapy (CRT) is an effective tool for local control of rectal cancer, but the disease recurrence rate remains high. The aim of this study was to retrospectively evaluate the correlation between PD-L1 expression and clinicopathological variables in rectal cancer after neoadjuvant CRT.
Materials and methods
A total of 90 rectal cancer patients who underwent neoadjuvant CRT were enrolled in this study. We evaluated PD-L1 expression using immunohistochemistry. Moreover, we investigated the correlation between PD-L1 expression and tumor-infiltrating T cells, and between CD8- and Foxp3-positive cells.
Results
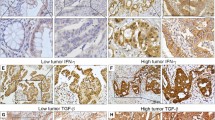
Patients with high PD-L1 expression more frequently had vascular invasion and tumor recurrence compared to patients with low PD-L1 expression (P = 0.0225 and P = 0.0051). High PD-L1 expression was significantly associated with poor recurrence-free and overall survival (P = 0.0027 and P = 0.0357). Multivariate analysis revealed lymph node metastasis and high PD-L1 expression as independent risk factors for tumor recurrence (P = 0.0102 and P = 0.0374). Numbers of infiltrating CD8-positive cells in patients with high PD-L1 expression were significantly lower than in patients with low PD-L1 expression (P = 0.0322).
Conclusion
Our data suggest that inhibition of PD-L1 may be a new immunotherapeutic strategy to reduce tumor recurrence and improve prognosis in patients with rectal cancer after neoadjuvant CRT.



Similar content being viewed by others
References
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
van den Brink M, Stiggelbout AM, van den Hout WB et al (2004) Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol 22:3958–3964
Guillem JG, Chessin DB, Cohen AM et al (2005) Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 241:829–836 discussion 836–828
Bosset JF, Collette L, Calais G et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Peeters KC, Marijnen CA, Nagtegaal ID et al (2007) The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 246:693–701
Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A (2014) Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 153:145–152
Dolan DE, Gupta S (2014) PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 21:231–237
Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242
Keir ME, Liang SC, Guleria I et al (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203:883–895
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Sabatier R, Finetti P, Mamessier E et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6:5449–5464
Shen JK, Cote GM, Choy E et al (2014) Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res 2:690–698
Curiel TJ, Wei S, Dong H et al (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–567
Zhang L, Gajewski TF, Kline J (2009) PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114:1545–1552
Iwai Y, Ishida M, Tanaka Y et al (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Hirano F, Kaneko K, Tamura H et al (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65:1089–1096
Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY (2008) PD-L1: PD-1 interaction contributes to the functional suppression of T-cell responses to human uveal melanoma cells in vitro. Invest Ophthalmol Vis Sci 49:2518–2525
Saigusa S, Inoue Y, Tanaka K et al (2013) Lack of M30 expression correlates with factors reflecting tumor progression in rectal cancer with preoperative chemoradiotherapy. Mol Clin Oncol 2:99–104
Ryan R, Gibbons D, Hyland JM et al (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47:141–146
Pages F, Berger A, Camus M et al (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Mori K, Toiyama Y, Saigusa S et al (2015) Systemic analysis of predictive biomarkers for recurrence in colorectal cancer patients treated with curative surgery. Dig Dis Sci 60:2477–2487
Amarnath S, Mangus CW, Wang JC et al (2011) The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med 3:111ra120
Salama P, Phillips M, Grieu F et al (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Yoon HH, Orrock JM, Foster NR et al (2012) Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One 7:e42274
Huang Y, Liao H, Zhang Y et al (2014) Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: a meta analysis. PLoS One 9:e94376
Shinto E, Hase K, Hashiguchi Y et al (2014) CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 21(Suppl 3):S414–S421
Tsuchikawa T, Md MM, Yamamura Y et al (2012) The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol 19:1713–1719
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Saigusa, S., Toiyama, Y., Tanaka, K. et al. Implication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapy. Int J Clin Oncol 21, 946–952 (2016). https://doi.org/10.1007/s10147-016-0962-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-0962-4