Abstract
Background
The aim of this retrospective study was to investigate the tumor characteristics, surgical details, and survival distribution of surgically resected cases of gastric cancer from the nationwide registry of the Japanese Gastric Cancer Association.
Methods
Data from 118,367 patients with primary gastric carcinoma who underwent resection between 2001 and 2007 were included in the survival analyses. The 5-year survival rates were calculated for various subsets of prognostic factors.
Results
The median age of the patients was 67 years. The proportions of patients with pathological stage (Japanese Gastric Cancer Association) IA, IB, II, IIIA, IIIB, and IV disease were 44.0%, 14.7%, 11.7%, 9.5%, 5.0%, and 12.4% respectively. The death rate within 30 days of operation was 0.5%. The 5-year overall survival rate in the 118,367 patients who were treated by resection was 71.1%. The 5-year overall survival rates of patients with pathological stage IA, IB, II, IIIA, IIIB, and IV disease were 91.5%, 83.6%, 70.6%, 53.6%, 34.8%, and 16.4% respectively. The 5-year disease-specific survival rates in the patients with pT1 (mucosa) disease after D1+ dissection of lymph node station no. 7 (D1 + α), D1+ dissection of lymph node station nos. 7, 8, and 9 (D1+ β), and D2 lymphadenectomy were 99.4%, 99.6%, and 99.1% respectively. The 5-year disease-specific survival rates in the patients with pT1 (submucosa) disease after D1 + α, D1 + β, and D2 lymphadenectomy were 97.3%, 98.1%, and 96.9% respectively.
Conclusion
Detailed analyses of the data from more than 100,000 patients show the recent trends of the outcomes of gastric cancer treatment in Japan and provide baseline information for use by medical communities around world.
Similar content being viewed by others
Introduction
A nationwide gastric cancer registration project was started in 1968 by the Japanese Research Society for Gastric Cancer. The society was reorganized in 1997 into the Japanese Gastric Cancer Association (JGCA), which performed detailed survival analyses of the patients registered from 1963 to 1991 and published the results [1]. After a 10-year period of inactivity, the registration committee of the JGCA rebooted the program in 2001. Herein, we report the treatment outcomes of gastric cancer patients in the registry who underwent surgical treatment between 2001 and 2007. Of particular note during this period is that the first version of the gastric cancer treatment guidelines was issued by the JGCA in 2001 [2], and the revised version was issued in 2004 [3].
The gastric cancer treatment guidelines recommend endoscopic treatment (endoscopic mucosal resection, endoscopic submucosal dissection) and gastrectomy with a reduced extent of lymphadenectomy than D2 as modified gastrectomy (D1 + α, D1 + β) for stage I disease. They also introduces optional treatment methods, such as pylorus-preserving gastrectomy (PPG), a vagus-nerve preserving method, and laparoscopic assistance.
The aim of this retrospective study was to review the tumor characteristics, surgical details, and survival distribution in surgically resected cases of gastric cancer in Japan to demonstrate the recent trends in the treatment results in Japanese hospitals, in general, to publish valuable information for use by medical communities around the world, and to provide baseline information for future clinical trials.
Patients and methods
Hospitals to which members of the JGCA are affiliated voluntarily downloaded and fulfilled the requirements for the database requested by the JGCA and sent the anonymized data from the patients 5 years after they had undergone surgery to the JGCA data center. Data on a total of 53 items, including the surgical procedures, pathological diagnosis, and the prognosis, pertaining to patients with primary gastric carcinoma treated surgically between 2001 and 2007 were collected retrospectively. The accumulated data from the patients were reviewed and analyzed by the JGCA Registration Committee according to previously reported methods [4, 5]. The definition and documentation of the items were based on the JGCA Japanese Classification of Gastric Carcinoma (13th edition) [6] and the Union for International Cancer Control TNM Classification of Malignant Tumors (fifth edition) [7].
The 5-year overall survival rates were calculated for various subsets of prognostic factors by the Kaplan–Meier method. Deaths from any cause observed during the 5-year postoperative period were counted as events in the survival analysis. The 5-year disease-specific survival rates were also calculated. Deaths from the primary disease during the 5-year postoperative period were counted as events in the survival analysis. Survival curves drawn with use of the Kaplan–Meier method were compared by the log-rank test. The P values were calculated by the log-rank test.
The nationwide registration program was approved by the Ethics Committee of the JGCA. Every hospital discloses information to the patients about the nationwide registry of the JGCA. Participating patients were excluded only when they specified that they were unwilling to participate.
Results
Between 2001 and 2007, 125,284 patients with primary gastric carcinoma were enrolled from 367 institutions in Japan. Of these, 1770 were excluded because the type of surgery was not specified in the data. Of the remaining patients, 120,202 underwent gastric resection and 3312 did not undergo resection. Accordingly, the resection rate was 97.3% (120,202/123,514). In all, 1835 patients had missing data (sex, age, vital status, survival period). After exclusion of these patients, the data from the remaining 118,367 patients who had undergone gastric resection were included in the survival analysis.
Of the 118,367 patients, 17,944 were lost to follow-up, yielding a follow-up rate of 84.8%. When the follow-up period was less than 1825 days, the patients were regarded as “lost to follow-up”. The 25th, 50th, and 75th percentile of the follow-up period in the lost to follow-up patients were 299, 881, and 1497 days after the operation respectively. The most frequent value was 1821 days.
The patient volumes in the participating hospitals are indicated in Fig. 1. The median patient volume was 58 patients per year. Five hospitals were high-volume centers, recording more than 200 cases per year.
The patient demographics are summarized in Table 1. The median patient age was 67 years. The proportion of patients who were older than 80 years was 9.5%. The male-to-female ratio was 2.2:1. In all, 74.0% of the tumors were located in the middle third or lower third of the stomach, with cancers in the upper third of the stomach accounting for only 21.7% of the cases. The most frequent macroscopic type was type 0 (53.1%), followed by type 3 (19.8%), type 2 (14.0%), and type 4 (6.6%). The ratio of the differentiated type of cancer to the undifferentiated type of cancer was 1.1:1. Early gastric cancer (T1) was confirmed pathologically in approximately 50% of the patients. The mucosa to submucosa ratio was 1:1. Of the total population, 40% of patients had nodal involvement, and approximately 20% had metastasis beyond pN2 (anatomical classification of the JGCA). Peritoneal washing cytology was performed in 50.2% of all patients, 75.7% of patients with pT3 (serosa exposed) disease, and 77.2% of patients with pT4 (serosa infiltrating) disease. The positive cytology findings rate was 29.7% in the patients with pT3 (serosa exposed) disease, 34.8% in the patients with pT4 (serosa infiltrating) disease, and 11.9% in the overall population. Synchronous liver metastases were found in 2.2% of patients, and peritoneal seeding was found in 5.3% of patients. In all, 58.7% of the patients had pathological stage I disease, which was followed in frequency by pathological stage III (14.5%), stage IV (12.4%), and stage II (11.7%) disease.
The surgical procedures adopted and the outcomes are summarized in Table 2. Distal gastrectomy was performed in 60.2% of patients, PPG was performed in 3.4% of patients, total gastrectomy was preformed in 29.6% of patients, and proximal gastrectomy was performed in 4.6% of patients. Approximately 90% of patients underwent open surgery and approximately 10% underwent laparoscopic gastrectomy. The frequency of adoption of laparoscopic gastrectomy increased dramatically from 3.5% to 17.8% during this period (Fig. 2). The gallbladder (17.7%) and spleen (9.3%) were also frequently resected during the gastrectomy. D2 lymphadenectomy was performed in 47.1% of patients and was the predominant type of lymphadenectomy performed. Modified D1 + α or D1 + β gastrectomy was performed in 27.5% of patients. The proportion of patients undergoing modified (D1 + α or D1 + β) gastrectomy increased from 18.5% to 35.2% during this period (Fig. 2). Surgery with a sufficient surgical margin was completed in more than 95% of patients. R0 resection (curability A and B) was performed in 87.9% of patients.
The median number of dissected nodes was 28 in all the patients, 28 in those who underwent surgery via laparotomy, 35 in those who underwent surgery via thoracotomy, and 25 in those who underwent surgery via the laparoscopic approach. The median number of dissected node was 8 for D0 lymphadenectomy, 20 for D1 lymphadenectomy, 34 for D2 lymphadenectomy, 48 for D3 lymphadenectomy, 20 for D1 + α lymphadenectomy, and 28 for D1 + β lymphadenectomy.
Of the 118,367 patients who had undergone gastrectomy, 587 died within 30 days of surgery, yielding a 30-day operative mortality of 0.5%. Further, 1252 died within 60 days of surgery, yielding a 60-day operative mortality of 1.1%, and 2004 died within 90 days of surgery, yielding a 90-day operative mortality of 1.7%. In total, 31,812 patients died during the follow-up period. The most frequent cause of death in the patients who had undergone gastrectomy was death from the primary disease (n = 21,927), followed by death from other diseases (n = 5004) and other cancers (n = 2007). The predominant mode of recurrence was peritoneal metastasis (n = 7769), followed by hematogenous metastasis (n = 4526), lymph node metastasis (n = 2218), and local recurrence (n = 1378) (Table 3). When recurrence at unknown sites was excluded, the predominant mode of recurrence in the patients with pT1 tumor was hematogenous metastasis (47.2%, 406/861). In patients with pT3 or T4 tumors, the predominant mode of recurrence was peritoneal metastasis (59.4%, 6087/10,248), followed by hematogenous metastasis (21.3%, 2178/10,248).
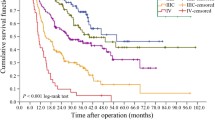
The 5-year overall survival rate in the 118,367 patients who had undergone resection of the primary gastric cancer was 71.1% (95% confidence interval 70.9–71.3%) (Table 1). There was a difference between males and females in the overall survival distribution, but not in the disease-specific survival distribution. In all, 2530 patients had tumors invading the esophagus. The 5-year overall survival rate was 45.6% (95% confidence interval 43.6–47.6%) and the 5-year disease-specific survival rate was 55.3% (95% confidence interval 53.1–57.5%) in these patients. Significant differences in the prognosis were observed among the disease stages classified according to the JGCA classification (Table 1, Fig. 3). However, subgroup analyses of the survival distribution in every stage by the pT and pN category (Table 4) revealed a lack of homogeneity in the 5-year overall survival rates among patients classified as having stage IB, II, IIIA, or IV disease.
The survival for each stage (JGCA) according to the age group is summarized in Table 5. Elderly patients showed poorer survivals, not only in terms of the overall survival but also in terms of disease-specific survival, for every stage of the disease.
To compare the outcomes of reduced-extent lymphadenectomy, reduced-extent gastrectomy, and standard D2 gastrectomy for early gastric cancer, the proportions of node-positive patients, the 5-year overall survival rates, and the 5-year disease-specific survival rates were calculated, as shown in Table 6.
Comparison of the patients who underwent D1, D1 + α, D1 + β, or D2 gastrectomy revealed a higher number of node-positive patients in the D2 gastrectomy group than in the other groups. In the pT1 (mucosa) patients, the 5-year overall survival rate of those treated by D1 or D1 + α gastrectomy was lower than that of those treated by D2 gastrectomy. On the other hand, the 5-year disease-specific survival rates were similar. In the pT1 (submucosa) patients, the 5-year overall survival rate of those who underwent D1 or D1 + α gastrectomy was lower than that of those who were treated by D2 gastrectomy. On the other hand, the 5-year disease-specific survival rate in the patients who underwent D2 gastrectomy was lower than that in patients who underwent D or /D1 + β gastrectomy.
There were more node-positive patients among those who had undergone distal gastrectomy (D2) than among those who had undergone PPG. Both the 5-year overall survival rate and the 5-year disease-specific survival rate after distal gastrectomy (D2) were lower than those after PPG. There were more node-positive patients among those who had undergone distal gastrectomy (D2) than among those who had undergone proximal gastrectomy. In the pT1 (mucosa) patients, the 5-year overall survival rate of those who underwent proximal gastrectomy was similar to that of those who were treated by D2 gastrectomy. In the pT1 (submucosa) patients, the 5-year overall survival rate of those treated by proximal gastrectomy was lower than that of those treated by D2 gastrectomy. On the other hand, the 5-year disease-specific survival rates were similar.
Discussion
The number of gastric cancer incidence cases in Japan between 2001 and 2007 was 785,949 [8]. The data from the 125,284 patients registered with this program accounted for the data from approximately 15% of the total estimated gastric cancer population in Japan.
The median age of the registered patients was 65.8 years; it was 62.0 years in 1990, and 57.9 years in 1975 [1]. The proportion of patients older than 80 years was 0.7% in 1963 [1], 4.5% in 1991 [9], and 9.5% in the period included in this study, representing a dramatic increase. The population represented in this gastric cancer registry is still aging. In regard to the life expectancy at birth, it was 71.73 years in 1975, 75.92 years in 1990, and 79.19 years in 2006 for men, and 76.89 years in 1975, 81.90 years in 1990, and 85.99 years in 2006 for women [10]. Thus, the increase in the average age in this study can be considered to be mainly due to aging of the Japanese population.
The proportion of patients with cancer involving the upper third of the stomach gradually increased from 12.9% in 1963 to 21.7% in 1991 [8, 9]. However, the proportion between 2001 and 2007 (20.7%) was similar to that in 1991. Several articles have reported an increase in the frequency of junctional tumors. However, from this registry it is not possible to discriminate junctional tumors, although tumors invading the esophagus could be identified. The proportion of gastric cancer patients with diagnosed early gastric cancer reached approximately 50% in the 1990s. It has been stable for surgical cases, however, considering the increase in the frequency of endoscopic treatment [11], and it could be concluded that the proportion of patients with diagnosed early gastric cancer is still increasing. The proportion of patients with a diagnosis of submucosal cancer among the early gastric cancer patients gradually decreased from 60% in 1963 to 40% in 1991. However, in this analysis, the proportion of patients with submucosal cancer was more than 50%. A possible reason is that a large number of patients with mucosal cancer were treated by endoscopy and not by surgery during this period, because the guidelines approved endoscopic treatment. The proportion of patients with pN0 disease was 30% in 1963, and has remained at 60% through the 1990s until now.
PPG was recommended in the guidelines as one of the surgical options for early gastric cancer. The penetration rate was 3.3%, still low. The frequency of adoption of laparoscopic gastrectomy increased dramatically from 2001 to 2007. Considering that the first laparoscopic distal gastrectomy was performed in 1999, this increase reflects the very high interest of surgeons in this new surgical field.
The proportion of patients undergoing modified gastrectomy also increased during this period. The most plausible reason is the widespread use of the guidelines, which defined modified gastrectomy. Second, D2 laparoscopic gastrectomy is technically demanding, so modified gastrectomy might be preferred when the laparoscopic approach is adopted.
For pT1 tumors, hematogenous recurrence represented the predominant mode of recurrence, consistent with other reports [12, 13]. When the tumor does not invade the serosa, peritoneal recurrence is rare. Lymph node recurrence could be well controlled by lymphadenectomy. However, hematogenous recurrence cannot be controlled by gastrectomy with lymphadenectomy.
The 5-year overall survival rate after resection was 68.2% in the nationwide registry of 1991 [9] and this has remained the same. The 5-year overall survival rate for every stage has also remained stable. Since 1991, there has been no increase in the proportion of patients with stage I disease, which is associated with a better prognosis among surgically resected cases. Surgeons have been attempting to increase the survival rates of patients with advanced tumors since the 1980s by applying extended surgery, but to no avail. Several phase III trials have failed to establish the validity of these extended procedures [14–16]. The validity of S-1 adjuvant chemotherapy for stage II and stage III disease was confirmed in 2007 [17]. Since S-1 therapy had not been established as standard treatment during most of the study period (2001–2007), we expect better outcomes in future analyses of data from this registry after 2008.
When we introduced reduced surgery, our concern was that this surgery should not prove inferior to D2 gastrectomy. Although the 5-year overall survival rate after D1 or D1 + α gastrectomy was lower than that after D2 gastrectomy, the 5-year disease-specific survival rate associated with D1 or D1 + α gastrectomy was not inferior to that associated with D2 gastrectomy. There is the possibility that D1 or D1 + α gastrectomy is often performed for frail patients, in whom surgeons hesitate to perform D2 gastrectomy. The 5-year disease-specific survival rates after D1, D1 + α, D1 + β gastrectomy, and PPG were rather better than the 5-year disease-specific survival rate after D2 gastrectomy. The reason might be that D2 gastrectomy is more often performed for more node-positive patients and the proportion of disease-specific deaths was higher than that after modified gastrectomy. Since the major limitations of this study relate to its retrospective design, we cannot justify the application of reduced surgery for pT1 tumors on the basis of the results of this study alone. Another limitation of this study was the low follow-up rate, although the 75th percentile of the follow-up period in the lost to follow-up patients was 1497 days. The data were retrospectively collected 7 years after the surgery, and the overall follow-up rate in our program was 84.7%, as already mentioned. A lower follow-up rate is generally considered to yield misleading results in terms of higher survival rates in patients with advanced disease.
Conclusion
Although the data were collected retrospectively and the follow-up rate was rather low, the results of detailed analyses of the data from more than 100,000 patients show the recent trends in the outcomes of gastric cancer treatment in Japan and provide baseline information for use by medical communities around the world.
References
Japanese Research Society for Gastric Cancer, Japanese Gastric Cancer Association. Zenkoku igan touroku chousa houkokusyo (Statstical report of nationwide registry of gastric carcinoma), no. 1–54. Tokyo: National Cancer Center; 1972–2001.
Japanese Gastric Cancer Association. Introduction to JGCA gastric cancer treatment guidelines. 2001. http://www.jgca.jp/pdf/E-guideline.pdf. Accessed 10 April 2017
Japanese Gastric Cancer Association. Guidelines for diagnosis and treatment of carcinoma of the stomach April 2004 edition. Edited by the Japanese Gastric Cancer Society. http://www.jgca.jp/pdf/Guidelines2004_eng.pdf. Accessed 10 April 2017
Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14(4):301–16.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1–27.
Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24.
Sobin LH, Wittekind C, editors. TNM classification of malignant tumors. 5th ed. New York: Wiley-Liss; 1997.
Cancer Information Service, National Cancer Center, Japan. Cancer Incidence. 2011;1975–2012. Cancer Statistics in Japan, Table download. http://ganjoho.jp/en/professional/statistics/table_download.html. Accessed 10 April 2017
Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:21–66.
Statistics and Information Department, Minister’s Secretariat, Ministry of Health, Labour and Welfare, Japanese Government. Abridged life tables for Japan 2010. http://www.mhlw.go.jp/english/database/db-hw/lifetb10/index.html. Accessed 10 April 2017
Tanabe S, Hirabayashi S, Oda I, Ono H, Nashimoto A, Isobe Y, et al. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017. doi:10.1007/s10120-017-0699-4.
Yuasa N, Nimura Y. Survival after surgical treatment of early gastric cancer, surgical techniques, and long-term survival. Langenbecks Arch Surg. 2005;390(4):286–93.
Wu B, Wu D, Wang M, Wang G. Recurrence in patients following curative resection of early gastric carcinoma. J Surg Oncol. 2008;98(6):411–4.
Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7(8):644–51.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–62.
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265(2):277–83.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Acknowledgements
The Japanese Gastric Cancer Association Registration Committee appreciates the great effort of the participating hospitals in registering accurate and detailed data for this project. We wish to especially express our sincere gratitude to Yuki Yamamoto, Niigata University Medical and Dental Hospital, for her valuable assistance.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
The authors declare that this study was not funded.
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and in compliance with the Helsinki Declaration of 1964 and later versions. Every hospital discloses information to the patients about the nationwide registry of the Japanese Gastric Cancer Association. Participating patients were excluded only when they specified that they were unwilling to participate.
Rights and permissions
About this article
Cite this article
Katai, H., Ishikawa, T., Akazawa, K. et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer 21, 144–154 (2018). https://doi.org/10.1007/s10120-017-0716-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-017-0716-7