Abstract
Most accepted definitions of reactive arthritis (ReA) consider it a type of spondyloarthritis (SpA) precipitated by a gut or urogenital infection. A wider definition considers any arthritis that occurs after a mucosal surface infection as ReA. There is limited consensus regarding a working definition, status of HLA-B27, or even classification criteria for ReA. This may also contribute to a lack of systemic studies or clinical trials for ReA, thereby reducing further treatment recommendations to expert opinions only. The emergence of post-COVID-19 ReA has brought the focus back on this enigmatic entity. Post-COVID-19 ReA can present at extremes of age, appears to affect both sexes equally and can have different presentations. Some present with small joint arthritis, others with SpA phenotype-either with peripheral or axial involvement, while a few have only tenosynovitis or dactylitis. The emergence of post-vaccination inflammatory arthritis hints at similar pathophysiology involved. There needs to be a global consensus on whether or not to include all such conditions under the umbrella of ReA. Doing so will enable studies on uniform groups on how infections precipitate arthritis and what predicts chronicity. These have implications beyond ReA and might be extrapolated to other inflammatory arthritides.
Key Points |
• Classical reactive arthritis (ReA) has a spondyloarthritis phenotype and is preceded by symptomatic gut or urogenital infection |
• The demonstration of antigen and nucleic acid sequences of pathogens in synovium has blurred the difference between invasive arthritis and reactive arthritis |
• Post-COVID-19 ReA has a transient phenotype and can have different presentations. All reported cases are self-limiting |
• The large amount of literature reporting post-COVID-19 ReA calls for introspection if the existing definitions of ReA need to be updated. |
Similar content being viewed by others
Introduction
Reactive arthritis (ReA) is classically considered a sub-type of spondyloarthritis (SpA) that is precipitated after a gastrointestinal or genitourinary infection [1]. The usual presentation is monoarticular or oligoarticular arthritis involving large joints that occurs around 2–4 weeks after an infection [2]. However, the term has been used in a wider context of an immune-mediated arthritis that may occur after any infection. The primary concept is that there is no direct invasion of the joints by any pathogen but the arthritis occurs as a result of induced changes in the immune system.
The proposed definitions of ReA have under the umbrella of SpA, be it under the Amor or the European Spondyloarthropathy Study Group (ESSG) proposed criteria for “Spondyloarthropathy” [3] or the currently used ASAS (ASsessment in Ankylosing Spondylitis working group) criteria for peripheral SpA [4]. According to these definitions, the pathognomic features of SpA are required to label a patient as having ReA. These include sacroiliitis, uveitis, dactylitis, enthesitis, and HLA-B27 or family history of SpA, psoriasis, or uveitis [4, 5].
ReA allows us a distinctive opportunity to scrutinize and learn how an infective trigger precipitates an autoimmune phenomenon. A majority of ReA resolves within a few weeks to a few months. The rest assume a chronic form indistinguishable from other chronic autoimmune arthritides [6]. Thus, it also provides an opening to understand how the autoimmune process becomes self-sustaining and chronic.
ReA is a predominant problem of low-to-middle income countries where gut and urinary tract infections abound. Though it is reported from high-income countries, the phenotype is usually limited to arthralgia, tenosynovitis, dactylitis or often not-so-severe arthritis. The phenotype seen in the tropics is much different with the rapid development of secondary osteoarthritis or even evolution into ankylosing spondylitis [7]. However, with the COVID-19 pandemic, there are a lot of reports of post-COVID-19 ReA, re-igniting interest in this entity worldwide.
This perspective aims to explore how the concept of ReA has evolved over the last century, touching upon similar entities and finally how the COVID-19 pandemic is coercing us to re-look into the definitions of this enigmatic malady.
Search strategy
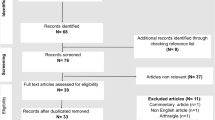
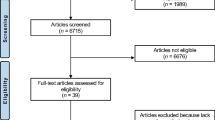
We have adhered to recommendations for narrative review searches [8]. We searched through Scopus and LitCovid/PubMed databases [9]. Non-English sources have not been consulted. Conference abstracts or non-peer reviewed sources were not included. To avoid confusion, we used the MeSH keyword “reactive arthritis” that includes “post infectious arthritis” for searches through LitCovid/PubMed. For Scopus, we used “reactive arthritis” OR “post infectious arthritis” in the search string.
History of ReA
The first descriptions of a post-infectious arthritis were made during the time of the First World war by Fiessinger and Leroy [10]. However, it was more commonly known with the eponym from a Nazi doctor who had first described a triad of urethritis, conjunctivitis, and arthritis. However, since he was convicted of war crimes, the eponym is not encouraged [11]. Also, a similar triad had already been described almost a century ago by Sir Benjamin Brodie in five cases [12].
More than half a century after the First World War, the concept of ReA was established as a non-purulent arthritis that occurred after a gastrointestinal infection without the direct invasion of the bacteria into the joints [13]. This concept was first contradicted by the finding of Chlamydia elementary bodies in the synovial cells of patients with ReA [14]. The tug of war over this concept has kept on going for a few decades. Now, it is clear that the entire live organism is not found in the joint but some antigen or genetic material, possibly carried by endosomes, may persist in the joint and lead to a sustained inflammatory reaction [15].
Current definitions and limitations
As the definition of ReA evolved, more and more entities were proposed for inclusion such as Lyme disease, gonococcal arthritis, post-streptococcal reactive arthritis, and rheumatic fever [16]. While it is true that Lyme disease and gonococcal arthritis may not fulfil the classical Koch’s postulates to be defined as an “infection,” both have unique characteristics clinical features. Clubbing them with ReA will neither help in the management nor further research. Similarly, the differences between ReA and post-streptococcal reactive arthritis are elaborated elsewhere [17].
The most commonly used definition of ReA has been provided by Braun and associates [18, 19]. This definition requires monoarthritis or oligoarthritis preceded by symptomatic diarrhoea or urethritis. For “definite” ReA to be diagnosed by the Braun criteria, an organism with known association with ReA needs to be demonstrated by culture or PCR. Even while these classification criteria were formulated, there was a lack of agreement on various points like the relationship of HLA-B27 with ReA, the existence of ReA without arthritis, or whether it should include only spondyloarthritis presentations or any arthritis [18]. More and more organisms are being added to the list of potential precipitants of ReA [20]. Also, the definition by Braun et al. does not consider the entity of “post-vaccination ReA.”
The American College of Rheumatology (ACR) or the European Alliance of Associations for Rheumatology(EULAR) do not have separate practice guidelines pertaining to ReA as possibly the rheumatologists in Europe or the United States do not see severe cases of ReA[21,22,23]. The incidence is apparently declining in most high-income countries [24]. However, the rest of the world that depend on the ACR and EULAR recommendations may find this gap challenging. For example, Latin America had the largest proportion of patients with “peripheral spondyloarthritis” [25]. ReA from India has arthritis as the predominant feature in 95% of patients [26] while a report from Finland showed only arthralgia in two and arthritis in none of 17 patients with post-Escherichia coli musculoskeletal conditions [23]. Thus, there seem to be great differences in how clinicians from different parts of the world view ReA.
Only a small percentage of patients who have infections with organisms such as Campylobacter, Salmonella, Shigella, or Yersinia develop ReA [27]. Similarly, amongst millions who have developed SARS-CoV-2 infection, only a minor proportion develops arthritis. Understanding this may help unearth new verities about the immune system and tolerance mechanisms.
Clinical phenotype of post-COVID-19 ReA
Phenotype
Post COVID-19 arthritis more commonly has a rheumatoid like phenotype affecting the wrists, ankles, and small joints of hands and feet. However, a spondyloarthritis-like presentation with axial involvement has also been reported [28]. It can also present as classical ReA with lower limb predominant oligoarthritis [29]. Isolate monoarthritis of a single metacarpophalangeal joint has also been reported [30]. Table 1 summarizes the different phenotypes, treatments given, and outcomes in various case reports of post-COVID-19 reactive arthritis from across the world.
Age and gender
The initial reports of post-COVID-19 ReA were in men past 50 years of age [31,32,33, 35]. This is in contrast to the classical ReA that is most common between 15 and 40 years of age. Again, at least three cases of post-COVID-19 ReA have also been reported in the paediatric age group [41, 45]. Unlike classical ReA, gender distribution appears equal between males and females. However, the total number of reported cases is too small for conclusive comments.
Treatment and outcome
The majority of the patients had responded to non-steroidal anti-inflammatory drugs (NSAIDs) while some received intra-articular steroids or rapidly tapered oral steroids (Table 2). Where outcomes are reported, usually, there was a response within the first week and the steroids /NSAIDs could be tapered down after 4 weeks. Only patients with rheumatoid arthritis-like phenotype with anti-citrullinated peptide antibodies had a chronic course and had to be given methotrexate [48,49,50].
Thus, the phenotype and outcomes of post-COVID-19 ReA appear to be different from those of classical ReA. These differences are summarized in Table 2.
Reactive arthritis after COVID-19 vaccination
Vaccination-induced autoimmunity is a concern since vaccines stimulate the immune system [51]. The first published case of ReA post-COVID-19 vaccination was reported in a 23-year-old woman after the inactivated Sinovac-CoronaVac vaccine [52]. We could identify a total of seven cases of inflammatory arthritis reported post-vaccination (Table 3).
Other post-COVID-19 inflammatory arthritis
We have reviewed post-COVID-19 rheumatic diseases at an earlier stage of the pandemic [57]. Post-COVID-19 peripheral nerve entrapment syndromes like carpal tunnel or tarsal tunnel syndromes have been hypothesized to be either due to localized demyelination, microangiopathy involving the vasa nervosum or an immune phenomenon targeting the adjacent synovial sheath [58]. An interesting group is the patients who have clinical phenotype and antibodies suggestive of rheumatoid arthritis developing post-COVID-19. These patients developed anti-cyclic citrullinated peptide antibody-positive arthritis after documented COVID-19 infection [48,49,50].
One concern was whether vaccination would cause a flare in persons with pre-existing autoimmune diseases [51]. Cases with flares of RA temporarily related to vaccination have been reported [59]. However, in a cohort of 724 patients with autoimmune rheumatic disease, only 4 patients had complained of a flare in joint pain. This was managed with NSAIDs and lasted less than a week [60].
In a cohort of 5493 RA patients from Hong Kong, a propensity-score weighted multivariate analysis did not show any association with COVID-19 vaccination and flare of RA [61].
Chronic arthritis after other viral infections
Several viruses are associated with acute polyarthritis that lasts less than 6–8 weeks [62]. In a small proportion of cases, such viral arthritis may become chronic such as in the case of HIV (Human Immunodeficiency Virus), Hepatitis B and C viruses [63, 64], parvovirus B19, and Chikungunya [65]. Some authors have argued that it may be better to label “COVID-19 associated arthritis” rather than “COVID-19 ReA” [66]. COVID-19 can also possibly precipitate arthritis in a susceptible individual. There is a case report of a lady with psoriasis and inflammatory bowel disease who developed arthritis post-COVID-19 infection [67].
Post-chikungunya or Parvovirus B-19 there can be an onset of arthritis indistinguishable from rheumatoid arthritis [68, 69]. A similar phenomenon has been reported post-COVID-19 too [48,49,50]. However, such anti-citrullinated antibody-positive RA has been reported only in 3 cases to date. The possibility of a coincidence cannot be excluded looking at the high incidence of COVID-19 infections and the not uncommon incidence of RA, but the point in support of a “reactive” arthritis is that the arthritis is seen after the acute COVID-19 infection. It is self-limiting. Had it been a direct viral arthritis, the synovitis should have occurred during the seroconversion phase. In acute COVID-19 infection, though arthralgia is common, documented arthritis has been rarely reported.
Possible pathogenic mechanisms
Viruses have been long implicated in the breakdown of immune tolerance and precipitation of autoimmune disease [70]. SARS-CoV-2 activates CD14 + monocytes and PD-L1 + neutrophils via the Osteopontin-mediated inhibition of Interleukin-10. This pathway is involved in rheumatoid arthritis and thus provides a common pathway for the evolution of inflammatory arthritis [71]. In Chikungunya viral infection, a prominent role of monocytes and anti-viral responses such as interferons has been postulated [72].
Interferon (IFN)-related pathways have been implicated in COVID-19 [73, 74] and these have a role in the initiation of rheumatoid arthritis. The TNF (Tumor Necrosis Factor)-induced animal models of rheumatoid arthritis are dependent on IFN and IFN response elements such as the IRF1 (interferon regulatory factor 1) transcription factor [75].
Also, various autoantibodies have been reported in COVID-19 [76]. Some of these might have pathological potential and if they persist after the infection, they may lead to rheumatic manifestations like arthritis. At least 15 different autoantibodies have been described in COVID-19 and 34 human peptides have similarities with SARS-CoV-2 proteins [77]. This may have implications for molecular mimicry in COVID-19.
Timelines of classic and post-COVID-19 reactive arthritides
Classical ReA is self-limiting in two-thirds of cases, but can damage the joints even in such a short period. Chronic ReA can have much worse sequelae. In the case of post-COVID-19 ReA, the manifestations appear more transient and self-limiting. This appears more similar to post-streptococcal ReA rather than classical ReA [17]. Also, some cases of post-COVID-19 ReA have different antibodies. There is a possibility that these may evolve into classifiable rheumatic diseases such as rheumatoid arthritis or lupus [57].
It is not necessary that all arthritis occurring post-COVID-19 should be reactive arthritis. The alternative is that it may be late-onset viral arthritis with actual invasion of the synovial space with the virus [78]. We could identify one study that reported the detection of SARS-CoV-2 RNA in a patient with wrist arthritis that had appeared 15 days after diarrhoea and upper respiratory tract symptoms [79]. However, other cases have not found such evidence [80]. Moreover, a post-mortem study also failed to find any viral RNA in synovial fluid or bone tissue in five patients who had died of COVID-19 [81].
Limitations
One limitation of this review is that the search strategy could miss cases of SARS-CoV-2 associated arthritis if the words “reactive” or “post-infectious” were not used. However, the main focus of the review was to assess how clinicians perceive and use the concept of reactive arthritis rather than only assessing SARS-CoV-2 associated arthritis.
Refining definitions for ReA
The definitions of ReA have been evolving gradually over the last half-century. Nevertheless, an ideal working definition still eludes us. Since this entity is not very common in high-income countries, there are possibly limited guidelines for this entity. The evidence base for treatment is also weak. The first and foremost requirement to fill in these deficiencies is a strong and universal definition of ReA.
Though there is a definite association between COVID-19 and arthritis, the scientific rigor to establish causality is incomplete yet. Thus, any new definition should allow for reasonable doubt, but still be sufficiently solid to further studies in the field.
The advent of ultrasound in the detection of enthesitis can enable a more objective definition [82]. Also, radiographic features such as new bone formation at the site of enthesitis can be a possible marker [83]. Radiographic changes are late but ultrasound diagnosis can be early with validated OMERACT (Outcome Measures in Rheumatology Clinical Trials) definitions available [84].
Conclusion
The emergence of post-COVID-19 ReA and possibly post-vaccination ReA is forcing a paradigm shift in how we perceive this entity. Post-vaccination autoimmune diseases are being reported [85]. This leads to the question of whether individuals with genetic predisposition such as HLA-B27 positivity need to be segregated for different vaccines [52].
As the SARS-CoV-2 pandemic is transformed into an endemic due to wide-spread vaccination and emergence of less virulent strains, it will be interesting to study how this affects emergence of COVID-19 associated autoimmune conditions including ReA.
Finally, post-infectious arthritis may hold the key to understanding how the chronicity of arthritis develops. This may help in future preventive strategies. The first step has to be a coordinated effort across nations and various rheumatology societies to set up working definitions and enumerate thrust areas of research for ReA.
References
García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, Iraheta I (2018) Reactive arthritis: update 2018. Clin Rheumatol 37:869–874. https://doi.org/10.1007/s10067-018-4022-5
Hannu T, Inman R, Granfors K, Leirisalo-Repo M (2006) Reactive arthritis or post-infectious arthritis? Best Pract Res Clin Rheumatol 20:419–433. https://doi.org/10.1016/j.berh.2006.02.003
Rosenbaum JT (2016) Evolving diagnostic criteria for axial spondyloarthritis. Ocul Immunol Inflamm 24:445–449. https://doi.org/10.3109/09273948.2016.1158277
Rudwaleit M, van der Heijde D, Landewé R et al (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70:25–31. https://doi.org/10.1136/ard.2010.133645
Dougados M, van der Linden S, Juhlin R et al (1991) The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 34:1218–1227
Kaarela K, Jäntti JK, Kotaniemi KM (2009) Similarity between chronic reactive arthritis and ankylosing spondylitis.A 32–35-year follow-up study. Clin Exp Rheumatol 27:325–328
Misra R, Ahmed S, Chaudhury A et al (2018) THU0269 Development of ankylosing spondylitis in patients with reactive arthritis and peripheral spondyloarthropathy: hospital based study in north India. Ann Rheum Dis 77:353–353. https://doi.org/10.1136/annrheumdis-2018-eular.6558
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Chen Q, Allot A, Lu Z (2020) Keep up with the latest coronavirus research. Nature 579:193–193. https://doi.org/10.1038/d41586-020-00694-1
Antonio I-G, Jose FR, Rafael V, Eric LM (2004) A brief history of Stoll-Brodie-Fiessinger-Leroy syndrome (Reiters syndrome) and reactive arthritis with a translation of Reiters original 1916 article into English. Curr Rheumatol Rev 1:71–79
Panush RS, Paraschiv D, Dorff REN (2003) The tainted legacy of Hans Reiter. Semin Arthritis Rheum 32:231–236. https://doi.org/10.1053/sarh.2003.49997
Brodie BC (1819) Pathological and surgical observations on diseases of the joints. Edinb Med Surg J 15:440–446
Ahvonen P, Sievers K, Aho K (1969) Arthritis associated with Yersinia enterocolitica infection. Acta Rheumatol Scand 15:232–253. https://doi.org/10.3109/rhe1.1969.15.issue-1-4.32
Gordon FB, Quan AL, Steinman TI, Philip RN (1973) Chlamydial isolates from Reiter’s syndrome. Br J Vener Dis 49:376–380. https://doi.org/10.1136/sti.49.4.376
Colmegna I, Cuchacovich R, Espinoza LR (2004) HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev 17:348–369. https://doi.org/10.1128/CMR.17.2.348-369.2004
Kuipers JG, Köhler L, Zeidler H (1999) Reactive or infectious arthritis. Ann Rheum Dis 58:661–664. https://doi.org/10.1136/ard.58.11.661
Ahmed S, Padhan P, Misra R, Danda D (2021) Update on post-streptococcal reactive arthritis: narrative review of a forgotten disease. Curr Rheumatol Rep 23:19. https://doi.org/10.1007/s11926-021-00982-3
Braun J, Kingsley G, van der Heijde D, Sieper J (2000) On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3–6, 1999. J Rheumatol 27:2185–2192
Selmi C, Gershwin ME (2014) Diagnosis and classification of reactive arthritis. Autoimmun Rev 13:546–549. https://doi.org/10.1016/j.autrev.2014.01.005
Zeidler H, Hudson AP (2021) Reactive arthritis update: spotlight on new and rare infectious agents implicated as pathogens. Curr Rheumatol Rep 23:53. https://doi.org/10.1007/s11926-021-01018-6
Townes JM (2010) Reactive arthritis after enteric infections in the United States: the problem of definition. Clin Infect Dis 50:247–254. https://doi.org/10.1086/649540
Courcoul A, Brinster A, Decullier E et al (2018) A bicentre retrospective study of features and outcomes of patients with reactive arthritis. Joint Bone Spine 85:201–205. https://doi.org/10.1016/j.jbspin.2017.01.013
Tuompo R, Lääveri T, Hannu T et al (2020) Reactive arthritis and other musculoskeletal symptoms associated with acquisition of diarrhoeagenic Escherichia coli (DEC). Ann Rheum Dis 79:605–611. https://doi.org/10.1136/annrheumdis-2019-216736
Hayes KM, Hayes RJP, Turk MA, Pope JE (2019) Evolving patterns of reactive arthritis. Clin Rheumatol 38:2083–2088. https://doi.org/10.1007/s10067-019-04522-4
López-Medina C, Molto A, Sieper J et al (2021) Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: results of the worldwide, cross-sectional ASAS-PerSpA study. RMD Open 7:e001450. https://doi.org/10.1136/rmdopen-2020-001450
Thomas KN, Anuja AK, Gupta L (2020) Clinical profile of adults and children with reactive arthritis in India - A cohort study. Indian J Rheumatol 15:304–309. https://doi.org/10.4103/injr.injr_126_20
Pogreba-Brown K, Austhof E, Tang X et al (2021) Enteric pathogens and reactive arthritis: systematic review and meta-analyses of pathogen-associated reactive arthritis. Foodborne Pathog Dis 18:627–639. https://doi.org/10.1089/fpd.2020.2910
Coath FL, Mackay J, Gaffney JK (2021) Axial presentation of reactive arthritis secondary to Covid-19 infection. Rheumatology (Oxford) keab009. https://doi.org/10.1093/rheumatology/keab009
Sureja NP, Nandamuri D (2021) Reactive arthritis after SARS-CoV-2 infection. Rheumatol Adv Pract 5:rkab001. https://doi.org/10.1093/rap/rkab001
Cincinelli G, Di Taranto R, Orsini F et al (2021) A case report of monoarthritis in a COVID-19 patient and literature review: simple actions for complex times. Medicine (Baltimore) 100:e26089. https://doi.org/10.1097/MD.0000000000026089
Saricaoglu EM, Hasanoglu I, Guner R (2021) The first reactive arthritis case associated with COVID-19. J Med Virol 93:192–193. https://doi.org/10.1002/jmv.26296
Liew IY, Mak TM, Cui L et al (2020) A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 26:233. https://doi.org/10.1097/RHU.0000000000001560
Ono K, Kishimoto M, Shimasaki T et al (2020) Reactive arthritis after COVID-19 infection. RMD Open 6:e001350. https://doi.org/10.1136/rmdopen-2020-001350
Talarico R, Stagnaro C, Ferro F et al (2020) Symmetric peripheral polyarthritis developed during SARS-CoV-2 infection. Lancet Rheumatol 2:e518–e519. https://doi.org/10.1016/S2665-9913(20)30216-2
Gasparotto M, Framba V, Piovella C et al (2021) Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol 40:3357–3362. https://doi.org/10.1007/s10067-020-05550-1
Colatutto D, Sonaglia A, Zabotti A et al (2021) Post-COVID-19 arthritis and sacroiliitis: natural history with longitudinal magnetic resonance imaging study in two cases and review of the literature. Viruses 13:1558. https://doi.org/10.3390/v13081558
Salvatierra J, Martínez-Peñalver D, Salvatierra-Velasco L (2020) CoVid-19 related dactyitis. Joint Bone Spine 87:660. https://doi.org/10.1016/j.jbspin.2020.06.009
Yokogawa N, Minematsu N, Katano H, Suzuki T (2020) Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis Annrheumdis-2020–218281. https://doi.org/10.1136/annrheumdis-2020-218281
Danssaert Z, Raum G, Hemtasilpa S. Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus 12:e9698. https://doi.org/10.7759/cureus.9698
Schenker HM, Hagen M, Simon D et al (2021) Reactive arthritis and cutaneous vasculitis after SARS-CoV-2 infection. Rheumatology (Oxford) 60:479–480. https://doi.org/10.1093/rheumatology/keaa689
Houshmand H, Abounoori M, Ghaemi R, et al (2020) Ten-year-old boy with atypical COVID-19 symptom presentation: a case report. Clin Case Rep [Online ahead of print]. https://doi.org/10.1002/ccr3.3521
Jali I (2020) Reactive arthritis after COVID-19 infection. Cureus 12:e11761. https://doi.org/10.7759/cureus.11761
Di Carlo M, Tardella M, Salaffi F (2021) Can SARS-CoV-2 induce reactive arthritis? Clin Exp Rheumatol 39(Suppl 128):25–26
Hønge BL, Hermansen M-LF, Storgaard M (2021) Reactive arthritis after COVID-19. BMJ Case Rep 14:e241375. https://doi.org/10.1136/bcr-2020-241375
Sinaei R, Pezeshki S, Parvaresh S et al (2021) Post SARS-CoV-2 infection reactive arthritis: a brief report of two pediatric cases. Pediatr Rheumatol Online J 19:89. https://doi.org/10.1186/s12969-021-00555-9
Shokraee K, Moradi S, Eftekhari T et al (2021) Reactive arthritis in the right hip following COVID-19 infection: a case report. Trop Dis Travel Med Vaccines 7:18. https://doi.org/10.1186/s40794-021-00142-6
Kocyigit BF, Akyol A (2021) Reactive arthritis after COVID-19: a case-based review. Rheumatol Int 41:2031–2039. https://doi.org/10.1007/s00296-021-04998-x
Baimukhamedov C, Barskova T, Matucci-Cerinic M (2021) Arthritis after SARS-CoV-2 infection. Lancet Rheumatol 3:e324–e325. https://doi.org/10.1016/S2665-9913(21)00067-9
Derksen VFAM, Kissel T, Lamers-Karnebeek FBG, et al (2021) Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis Annrheumdis 2021–219859. https://doi.org/10.1136/annrheumdis-2021-219859
Roongta R, Chattopadhyay A, Ghosh A (2021) Correspondence on “Onset of rheumatoid arthritis after COVID-19: coincidence or connected?” Ann Rheum Dis Annrheumdis 2021–220479. https://doi.org/10.1136/annrheumdis-2021-220479
Velikova T, Georgiev T (2021) SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int 41:509–518. https://doi.org/10.1007/s00296-021-04792-9
An Q-J, Qin D-A, Pei J-X (2021) Reactive arthritis after COVID-19 vaccination. Hum Vaccin Immunother 17:2954–2956. https://doi.org/10.1080/21645515.2021.1920274
Unal Enginar A (2021) Arthritis following COVID-19 vaccination: report of two cases. Int Immunopharmacol 101:108256. https://doi.org/10.1016/j.intimp.2021.108256
(2022) SARS-COV-2-vaccine-inactivated-Sinovac-Biotech. Reactions Weekly 1890:229–229. https://doi.org/10.1007/s40278-022-09226-x
Baimukhamedov C (2021) Arthritis of the left elbow joint after vaccination against SARS-CoV-2 infection. Int J Rheum Dis 24:1218–1220. https://doi.org/10.1111/1756-185X.14202
Baimukhamedov C, Makhmudov S, Botabekova A (2021) Seropositive rheumatoid arthritis after vaccination against SARS-CoV-2 infection. Int J Rheum Dis 24:1440–1441. https://doi.org/10.1111/1756-185X.14220
Ahmed S, Zimba O, Gasparyan AY (2021) COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol 40:2611–2619. https://doi.org/10.1007/s10067-021-05691-x
Roncati L, Gianotti G, Gravina D et al (2021) Carpal, cubital or tarsal tunnel syndrome after SARS-CoV-2 infection: A causal link? Med Hypotheses 153:110638. https://doi.org/10.1016/j.mehy.2021.110638
Terracina KA, Tan FK (2021) Flare of rheumatoid arthritis after COVID-19 vaccination. The Lancet Rheumatology 3:e469–e470. https://doi.org/10.1016/S2665-9913(21)00108-9
Cherian S, Paul A, Ahmed S et al (2021) Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int 41:1441–1445. https://doi.org/10.1007/s00296-021-04917-0
Li X, Tong X, Yeung WWY et al (2021) Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2021-221571
Marks M, Marks JL (2016) Viral arthritis Clin Med (Lond) 16:129–134. https://doi.org/10.7861/clinmedicine.16-2-129
Chen Y-L, Jing J, Mo Y-Q et al (2018) Presence of hepatitis B virus in synovium and its clinical significance in rheumatoid arthritis. Arthritis Res Ther 20:130. https://doi.org/10.1186/s13075-018-1623-y
Kemmer NM, Sherman KE (2010) Hepatitis C-related arthropathy: diagnostic and treatment considerations. J Musculoskelet Med 27:351–354
Tritsch SR, Encinales L, Pacheco N et al (2020) Chronic joint pain 3 years after Chikungunya virus infection largely characterized by relapsing-remitting symptoms. J Rheumatol 47:1267–1274. https://doi.org/10.3899/jrheum.190162
Kobayashi S, Taniguchi Y, Kida I, Tamura N SARS-CoV2-triggered acute arthritis: viral arthritis rather than reactive arthritis. Journal of Medical Virology n/a: https://doi.org/10.1002/jmv.27229
Novelli L, Motta F, Ceribelli A et al (2021) A case of psoriatic arthritis triggered by SARS-CoV-2 infection. Rheumatology (Oxford) 60:e21–e23. https://doi.org/10.1093/rheumatology/keaa691
Amaral JK, Bilsborrow JB, Schoen RT (2020) Chronic Chikungunya arthritis and rheumatoid arthritis: what they have in common. Am J Med 133:e91–e97. https://doi.org/10.1016/j.amjmed.2019.10.005
Takahashi Y, Murai C, Shibata S et al (1998) Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Natl Acad Sci USA 95:8227–8232
Smatti MK, Cyprian FS, Nasrallah GK et al (2019) Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses 11:762. https://doi.org/10.3390/v11080762
MacDonald L, Alivernini S, Tolusso B et al (2021) COVID-19 and RA share an SPP1 myeloid pathway that drives PD-L1+ neutrophils and CD14+ monocytes. JCI Insight 6:147413. https://doi.org/10.1172/jci.insight.147413
Suhrbier A (2019) Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat Rev Rheumatol 15:597–611. https://doi.org/10.1038/s41584-019-0276-9
Weisberg SP, Connors TJ, Zhu Y et al (2021) Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 22:25–31. https://doi.org/10.1038/s41590-020-00826-9
Ng KW, Faulkner N, Cornish GH et al (2020) Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 370:1339–1343. https://doi.org/10.1126/science.abe1107
Bonelli M, Dalwigk K, Platzer A et al (2019) IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes. Exp Mol Med 51:1–11. https://doi.org/10.1038/s12276-019-0267-6
Zhou Y, Han T, Chen J et al (2020) Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci 13:1077–1086. https://doi.org/10.1111/cts.12805
Dotan A, Muller S, Kanduc D et al (2021) The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20:102792. https://doi.org/10.1016/j.autrev.2021.102792
Jubber A, Moorthy A (2021) Reactive arthritis: a clinical review. J R Coll Physicians Edinb 51:288–297. https://doi.org/10.4997/JRCPE.2021.319
Kuschner Z, Ortega A, Mukherji P (2021) A case of SARS-CoV-2-associated arthritis with detection of viral RNA in synovial fluid. J Am Coll Emerg Physicians Open 2:e12452. https://doi.org/10.1002/emp2.12452
Yokogawa N, Minematsu N, Katano H, Suzuki T (2021) Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis 80:e101–e101. https://doi.org/10.1136/annrheumdis-2020-218281
Grassi M, Giorgi V, Nebuloni M et al (2021) SARS-CoV-2 in the knee joint: a cadaver study. Clin Exp Rheumatol. https://pubmed.ncbi.nlm.nih.gov/34665699/
Aguila Maldonado R, Ruta S, Valuntas ML, García M (2017) Ultrasonography assessment of heel entheses in patients with spondyloarthritis: a comparative study with magnetic resonance imaging and conventional radiography. Clin Rheumatol 36:1811–1817. https://doi.org/10.1007/s10067-017-3723-5
Jacobson JA, Girish G, Jiang Y, Resnick D (2008) Radiographic evaluation of arthritis: inflammatory conditions. Radiology 248:378–389. https://doi.org/10.1148/radiol.2482062110
Gandjbakhch F, Terslev L, Joshua F et al (2011) Ultrasound in the evaluation of enthesitis: status and perspectives. Arthritis Res Ther 13:R188. https://doi.org/10.1186/ar3516
Watad A, De Marco G, Mahajna H et al (2021) Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines 9:435. https://doi.org/10.3390/vaccines9050435
Author information
Authors and Affiliations
Contributions
All co-authors contributed substantially to the concept formulation, searches of relevant articles, and revisions. They approve the final version of the manuscript and take full responsibility for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
SA has received honorarium as speaker from Pfizer, DrReddy’s, Cipla, and Novartis (outside of the current work). All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bekaryssova, D., Yessirkepov, M., Zimba, O. et al. Reactive arthritis before and after the onset of the COVID-19 pandemic. Clin Rheumatol 41, 1641–1652 (2022). https://doi.org/10.1007/s10067-022-06120-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06120-3