Abstract
Purpose
The aim of this study was to evaluate the therapeutic effects of Anamorelin on patients with cancer anorexia-cachexia syndrome (CACS) based on a meta-analysis of published randomized trials.
Methods
We searched PubMed, Embase, Medline, and the Cochrane Central Register of Controlled Trials databases. Data from each selected study were evaluated individually. All continuous outcomes were calculated by the mean difference or standardized mean difference with 95% confidence interval for each study. Heterogeneity was assessed by using the Chi2 test at a significance level of P < 0.1, in addition to the I 2 statistic (I 2 > 50% indicated substantial heterogeneity).
Result
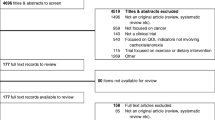
At last, four studies were included from 284 records. In three studies, lean body mass was reported and there was a significant difference between placebo and Anamorelin groups (P < 0.00001), without significant heterogeneity (I 2 = 0%). All the four studies reported the body weight change from baseline, and there was significant difference between placebo and Anamorelin groups (P = 0.007), but with high heterogeneity (I 2 = 97%). Two studies reported Anderson Symptom Assessment Scale (ASAS) score, and Anamorelin significantly increased the total ASAS score of CACS patients (P < 0.00001), without any heterogeneity (I 2 = 0%). Three studies reported non-dominant handgrip strength, and there was no significant difference between Anamorelin and placebo groups (P = 0.16). Three studies reported insulin-like growth factor-1 level, and there was significant difference between Anamorelin and placebo groups (P = 0.02), but with high heterogeneity (I 2 = 96%). Three studies reported IGF binding protein-3 concentration. Anamorelin significantly increased such concentration compared with placebo did (P < 0.00001). However, there was still higher heterogeneity (I 2 = 59%). All the included studies reported adverse events. Compared with placebo, Anamorelin induced fewer adverse events, but there was no significant difference between the two groups (RR = 0.07, P = 0.35).
Conclusion
In the included studies, Anamorelin had some positive effects to relieve the symptoms and improved the quality of life. However, the heterogeneity was apparent, so the clinical effects of Anamorelin should be further validated by increasing the sample size, varying the range of doses during treatment, and observing other outcomes. We are still confident for the future application of Anamorelin in phase III clinical trials.










Similar content being viewed by others
Abbreviations
- CACS:
-
Cancer anorexia-cachexia syndrome
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- RCT:
-
randomized controlled trial
- ASAS:
-
Anderson Symptom Assessment Scale
References
Tarricone R, Ricca G, Nyanzi-Wakholi B, Medina-Lara A (2016) Impact of cancer anorexia-cachexia syndrome on health-related quality of life and resource utilisation: a systematic review. Crit Rev Oncol Hematol 99:49–62
Inui A (2002) Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin 52:72–91
von Haehling S, Anker SD (2010) Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 1:1–5
Esposito A, Criscitiello C, Gelao L, Pravettoni G, Locatelli M, Minchella I, Di Leo M, Liuzzi R, Milani A, Massaro M, Curigliano G (2015) Mechanisms of anorexia-cachexia syndrome and rational for treatment with selective ghrelin receptor agonist. Cancer Treat Rev 41:793–797
Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A (2007) Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manag 34:94–104
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, O'Brien ME (2004) Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers. Br J Cancer 90:1905–1911
Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, Hopkinson J, Jacquelin-Ravel N, Jatoi A, Kaasa S, Strasser F (2014) Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 25:1492–1499
Mondello P, Mian M, Aloisi C, Fama F, Mondello S, Pitini V (2015) Cancer cachexia syndrome: pathogenesis, diagnosis, and new therapeutic options. Nutr Cancer 67:12–26
Madeddu C, Maccio A, Mantovani G (2012) Multitargeted treatment of cancer cachexia. Crit Rev Oncog 17:305–314
Shih A, Jackson KN (2007) Role of corticosteroids in palliative care. J Pain Palliat Care Pharmacother 21:69–76
Keifer JA, Guttridge DC, Ashburner BP, Baldwin AJ (2001) Inhibition of NF-κB activity by thalidomide through suppression of IκB kinase activity. J Biol Chem 276:22382–22387
Jin SH, Kim TI, Han DS, Shin SK, Kim WH (2002) Thalidomide suppresses the interleukin 1beta-induced NFkappaB signaling pathway in colon cancer cells. Ann N Y Acad Sci 973:414–418
Lundholm K, Korner U, Gunnebo L, Sixt-Ammilon P, Fouladiun M, Daneryd P, Bosaeus I (2007) Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res 13:2699–2706
Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K (2000) Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911
Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, Wang P (2007) Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med 176:805–813
Gonzalez-Rey E, Chorny A, Delgado M (2006) Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 130:1707–1720
Nagaya N, Kojima M, Kangawa K (2006) Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med 45:127–134
DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL (2007) Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 148:3004–3012
Guillory B, Splenser A, Garcia J (2013) The role of ghrelin in anorexia-cachexia syndromes. Vitam Horm 92:61–106
Morozumi N, Hanada T, Habara H, Yamaki A, Furuya M, Nakatsuka T, Inomata N, Minamitake Y, Ohsuye K, Kangawa K (2011) The role of C-terminal part of ghrelin in pharmacokinetic profile and biological activity in rats. Peptides 32:1001–1007
Leese PT, Trang JM, Blum RA, de Groot E (2015) An open-label clinical trial of the effects of age and gender on the pharmacodynamics, pharmacokinetics and safety of the ghrelin receptor agonist anamorelin. Clin Pharmacol Drug Dev 4:112–120
Garcia JM, Polvino WJ (2009) Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Hormon IGF Res 19:267–273
Garcia JM, Friend J, Allen S (2013) Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer 21:129–137
Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J (2015) Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. The Lancet Oncology 16:108–116
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 17:519–531
Takayama K, Katakami N, Yokoyama T, Atagi S, Yoshimori K, Kagamu H, Saito H, Takiguchi Y, Aoe K, Koyama A, Komura N, Eguchi K (2016) Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer 24:3495–3505
Chang VT, Hwang SS, Feuerman M (2000) Validation of the Edmonton symptom assessment scale. Cancer 88:2164–2171
Gale CR, Martyn CN, Cooper C, Sayer AA (2007) Grip strength, body composition, and mortality. Int J Epidemiol 36:228–235
Pietra C, Takeda Y, Tazawa-Ogata N, Minami M, Yuanfeng X, Duus EM, Northrup R (2014) Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. J Cachexia Sarcopenia Muscle 5:329–337
Northrup R, Kuroda K, Duus EM, Barnes SR, Cheatham L, Wiley T, Pietra C (2013) Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer 21:2409–2415
Zhang H, Garcia JM (2015) Anamorelin hydrochloride for the treatment of cancer-anorexia-cachexia in NSCLC. Expert Opin Pharmacother 16:1245–1253
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
Zhiqiang Zhao received the funding from the National Natural Science Foundation of China (No. 81373608), available from https://isisn.nsfc.gov.cn/egrantweb/.
Rights and permissions
About this article
Cite this article
Bai, Y., Hu, Y., Zhao, Y. et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support Care Cancer 25, 1651–1659 (2017). https://doi.org/10.1007/s00520-016-3560-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3560-0