Abstract
Purpose
Antiemetic guidelines recommend co-administration of targeted prophylactic medications inhibiting molecular pathways involved in emesis. NEPA is a fixed oral combination of a new NK1 receptor antagonist (RA), netupitant (NETU 300 mg), and palonosetron (PALO 0.50 mg), a pharmacologically distinct 5-HT3 RA. NEPA showed superior prevention of chemotherapy-induced nausea and vomiting (CINV) compared with oral PALO in a single chemotherapy cycle; maintenance of efficacy/safety over continuing cycles is the objective of this study.
Methods
This study is a multinational, double-blind study comparing a single oral dose of NEPA vs oral PALO in chemotherapy-naïve patients receiving anthracycline/cyclophosphamide-based chemotherapy along with dexamethasone 12 mg (NEPA) or 20 mg (PALO) on day 1. The primary efficacy endpoint was delayed (25–120 h) complete response (CR: no emesis, no rescue medication) in cycle 1. Sustained efficacy was evaluated during the multicycle extension by calculating the proportion of patients with overall (0–120 h) CR in cycles 2–4 and by assessing the probability of sustained CR over multiple cycles.
Results
Of 1455 patients randomized, 1286 (88 %) participated in the multiple-cycle extension for a total of 5969 cycles; 76 % completed ≥4 cycles. The proportion of patients with an overall CR was significantly greater for NEPA than oral PALO for cycles 1–4 (74.3 vs 66.6 %, 80.3 vs 66.7 %, 83.8 vs 70.3 %, and 83.8 vs 74.6 %, respectively; p ≤ 0.001 each cycle). The cumulative percentage of patients with a sustained CR over all 4 cycles was also greater for NEPA (p < 0.0001). NEPA was well tolerated over cycles.
Conclusions
NEPA, a convenient, guideline-consistent, fixed antiemetic combination is effective and safe over multiple cycles of chemotherapy.
Similar content being viewed by others
Introduction
International guideline committees consistently recommend combination antiemetic regimens targeting multiple molecular pathways associated with emesis as the standard of care for prevention of chemotherapy-induced nausea and vomiting (CINV) [1, 2]. Antiemetic guidelines currently recommend a prophylactic combination of a 5-HT3 receptor antagonist (RA), a neurokinin-1 (NK1) RA, and dexamethasone when administering highly emetogenic chemotherapy (HEC) or anthracycline-cyclophosphamide chemotherapy, as studies have shown a clear benefit with the addition of an NK1 RA to the standard 5-HT3 RA plus dexamethasone regimen in these settings [1, 2].
Unfortunately, adherence to antiemetic guidelines is suboptimal, despite continued research suggesting that guideline conformity improves CINV control for patients [3–5]. Consequently, even with effective agents available, many patients still suffer from CINV [6]. The supportive care community, in particular, continues to evaluate opportunities to reinforce and encourage implementation of guidelines into clinical practice, as well as monitor adherence.
Netupitant (NETU) is a new highly selective NK1 RA which has been developed as an oral fixed combination with palonosetron (referred to as NEPA; AKYNZEO®). Palonosetron was specifically chosen as the 5-HT3 RA for the NEPA combination because of its distinct pharmacological [7] and clinical [6] characteristics. Palonosetron is distinguished from the older 5-HT3 RAs in the class with its unique receptor binding, its ability to inhibit the cross talk between the 5-HT3 and NK1 receptors, its ability to induce NK1 receptor internalization and to work synergistically with NETU to enhance the inhibition of the substance P response, in addition to its distinctly better efficacy during the delayed (25–120 h) phase [7–9]. Consequently, it has the potential to enhance prevention of delayed CINV when used in combination with NETU. The NEPA combination also has the potential to improve guideline adherence by targeting two critical pathways involved in emesis with a convenient, single oral dose.
In a phase 2 single-cycle, dose-ranging study [10] in patients receiving HEC, the NEPA oral combination of NETU 300 mg + PALO 0.50 mg was shown to be superior to oral palonosetron during the delayed and overall (0–120 h) phases for all efficacy endpoints.
The current phase 3 study was designed to demonstrate superior prevention of CINV during cycle 1 with oral NEPA compared with oral palonosetron in patients receiving anthracycline-cyclophosphamide chemotherapy (and to evaluate NEPA’s safety). This study was also designed to evaluate whether the efficacy (and safety) seen in cycle 1 would be preserved over continuing treatment cycles. In a previously reported phase 3 study, NEPA was shown to be well tolerated over multiple cycles of HEC and moderately emetogenic chemotherapy (MEC) [11]. In the limited number of trials evaluating the multicycle efficacy of antiemetics, interpretation of the results has been challenging due to high dropout rates and differing statistical methods utilized [12]. As preservation of benefit over repeated cycles of chemotherapy is essential for optimal supportive care during cancer treatment, antiemetics need to be able to demonstrate a sustained benefit.
The cycle 1 data has been previously reported by Aapro et al. [13]; a single oral dose of NEPA plus dexamethasone prior to chemotherapy resulted in superior complete response (no emesis, no rescue medication) rates during the delayed phase (primary endpoint) compared with oral palonosetron plus dexamethasone. The efficacy of NEPA was supported by consistent superiority over oral palonosetron for all secondary efficacy endpoints (i.e., no emesis, no significant nausea, complete protection) during both the delayed and overall phases. This publication reports the results of the multiple-cycle extension of this study.
Materials and methods
Study design
This study was conducted between April 2011 and November 2012 in accordance with GCP, ICH, Declaration of Helsinki principles, and local laws and regulations. Protocol approval was obtained from ethical review committees for each site, and written informed consent was obtained from each patient before enrollment. The study design has been described in detail in the cycle 1 publication [13]. After completion of cycle 1, patients had the option to participate in a multiple-cycle extension, receiving the same treatment as assigned in cycle 1 for as long as they continued to fulfill the inclusion/exclusion criteria. There was no pre-specified limit of the number of repeat consecutive cycles. Patients received one of the following two treatments: oral NEPA (NETU 300 mg/PALO 0.50 mg) plus 12 mg dexamethasone or oral palonosetron 0.50 mg plus 20 mg dexamethasone, all given prior to chemotherapy each cycle.
Eligibility criteria
Eligible patients were ≥18 years, naïve to chemotherapy, and scheduled to receive their first course of an anthracycline/cyclophosphamide regimen for treatment of a solid malignant tumor. The chemotherapy consisted of either cyclophosphamide IV (500 to 1500 mg/m2) and doxorubicin IV (≥40 mg/m2) or cyclophosphamide IV (500 to 1500 mg/m2) and epirubicin IV (≥60 mg/m2). Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2. Patients were not eligible if they were scheduled to receive (1) HEC from days 1 to 5 or MEC from days 2 to 5 following chemotherapy, (2) radiation therapy to the abdomen or pelvis within 1 week prior to day 1 or between days 1 and 5, or (3) a bone marrow or stem cell transplant. Patients were not allowed to receive any drug with known or potential antiemetic efficacy within 24 h prior to day 1 and were excluded if they experienced any vomiting, retching, or mild nausea within 24 h prior to day 1. Patients were not to have had any serious cardiovascular disease history or predisposition to cardiac conduction abnormalities, with the exception of incomplete right bundle branch block. Because netupitant is a moderate inhibitor of CYP3A4, use of any CYP3A4 inducer within 4 weeks, use of a strong or moderate inhibitor within 1 week, or scheduled to receive CYP3A4 inhibitors, inducers, or certain substrates as concomitant medication were prohibited. For each cycle of the multiple-cycle extension, the investigator needed to consider it appropriate and not posing any unwarranted risk to the patient. There was to have been satisfactory study compliance in the preceding chemotherapy cycle, and patients were to be scheduled to receive the same chemotherapy regimen as cycle 1.
Assessments and statistical methods
From the start of chemotherapy infusion on day 1 through the morning of day 6 (0–120 h) of each cycle, patients completed a diary, capturing information pertaining to occurrence and timing of each emetic episode and rescue medication intake. Metoclopramide tablets were provided as rescue medication; however, the investigator was allowed to use an alternative rescue (excluding 5-HT3 or NK1 RAs, as well as palonosetron) at his/her discretion. The use of rescue medication for treatment of either nausea or vomiting was considered treatment failure. Severity of nausea was evaluated daily by patients in the diary by using a 100-mm horizontal visual analog scale (VAS). The left end of the scale (0 mm) was labeled as “no nausea,” and the right end of the scale (100 mm) was labeled as “nausea as bad as it could be.” No significant nausea was defined as a maximum score <25 mm.
The objective of the multiple-cycle extension was to compare the efficacy and safety of oral NEPA relative to oral palonosetron across multiple cycles of chemotherapy. The primary aim of the study was to demonstrate the superiority of NEPA over oral palonosetron based on the proportion of patients with a CR during the delayed (25–120 h) phase of cycle 1. In the multiple-cycle extension, the proportion of patients with CR and no significant nausea were prospectively defined efficacy endpoints, but no formal comparisons between groups were pre-specified. Thus, p values for the multiple-cycle extension should only be descriptively interpreted. CR, no emesis, and no significant nausea rates were compared by using a two-sided Cochran-Mantel-Haenszel (CMH) test including treatment, age class, and region as strata. A separate analysis of sustained CR evaluated the probability that a patient would remain a complete responder over 4 cycles of chemotherapy. This analysis was performed by using Kaplan-Meier methods, and patients who did not sustain a CR were considered treatment failures. Treatment groups were compared via a log-rank test. As the majority of patients had completed their planned chemotherapy after 4 cycles of treatment (as expected in “standard” AC protocols), only 36 % of patients received a fifth cycle and 27 % a sixth cycle; therefore, efficacy data is only presented through cycle 4.
Safety was assessed primarily by a clinical review of treatment-emergent adverse events. The multiple-cycle extension summarized incidence rates for cycles 2–6, while cycle 1 was evaluated separately. Cardiac safety was evaluated by cardiac adverse events, electrocardiogram (ECG) changes (including QTc), cardiac troponin levels, and left ventricular ejection fraction (LVEF). During each cycle, ECGs were recorded pre-dose and 5, 24, and 120 h post-dose. Troponin levels (cTnI) were measured pre-dose and 24 and 120 h post-dose of each cycle by using a standardized troponin assay (Siemens’ ADVIA Centaur TnI-Ultra troponin assay). A threshold of 0.12 ng/mL was considered an “alert value” [14]. LVEF was assessed for all patients at visit 1 and at the end of the study. No formal comparisons were performed for the safety assessments.
Results
A total of 1455 patients were randomized into the study. Of these, 1286 patients (88.4 %) entered the multiple-cycle extension, 167 patients did not enter the extension, and 4 patients did not receive the protocol-required chemotherapy or study drug in cycle 2 (Fig. 1). Patients participated in a total of 5969 chemotherapy cycles with 76 % of all patients (76 % NEPA, 77 % palonosetron) completing at least 4 cycles; 27 % of all patients completed 6 cycles.
Baseline and disease characteristics and emetic risk factors for patients entering the multiple-cycle extension are listed in Table 1 and are similar to those reported previously for patients in cycle 1. Treatment groups were similar and consistent with a cancer population receiving the protocol-specified anthracycline-cyclophosphamide chemotherapy regimen. Almost all patients were female with breast cancer (98 %); the majority were white (78 %), and the median age was 54 years. All patients but one (99.9 %) were treated with cyclophosphamide plus an anthracycline (65 % doxorubicin and 35 % epirubicin). Doses of these chemotherapeutic agents were similar at baseline and remained consistent for subsequent cycles.
Efficacy
The proportion of patients with an overall (0–120 h) CR was significantly greater for NEPA compared with oral palonosetron during cycle 1, and this was maintained in subsequent cycles (Fig. 2). The incremental benefit of NEPA over oral palonosetron in cycles 2–4 was greater than that seen in cycle 1 (7.7 % in cycle 1, 13.6 % in cycle 2, 13.5 % in cycle 3, and 9.2 % in cycle 4). CR rates were similar for NEPA and oral palonosetron during the acute phase but higher for NEPA compared with oral palonosetron during the delayed phase.
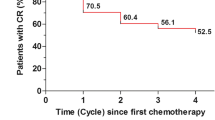
The percentage of patients who experienced a CR in cycle 1 and who sustained a CR over cycles 2–4 was significantly greater for NEPA than with oral palonosetron (p < 0.001, based on log-rank test) (Fig. 3). The absolute difference between NEPA and oral palonosetron in percentage of patients sustaining a CR continued to increase over time.
NEPA was also consistently more effective than oral palonosetron in preventing emesis and significant nausea over cycles 1–4 (Table 2). The percentage of patients who experienced no significant nausea in cycle 1 and who sustained this nausea control over cycles 2–4 was significantly greater for NEPA than oral palonosetron (p = 0.035, based on log-rank test) (Fig. 4).
Safety
Adverse events
The overall incidence, type, frequency, and intensity of treatment-emergent adverse events were comparable between the two treatment groups during the multiple-cycle extension (Table 3). There was no increase in incidence rates of adverse events across cycles, whether treatment-related or not (Table 4). Among the patients reporting adverse events during the multiple-cycle extension, the majority (85 %) reported adverse events of mild/moderate intensity. Of the 98 (15 %) NEPA-treated patients who experienced a severe adverse event, only 1 patient had a severe event considered to be treatment-related. The most common treatment-related adverse events were headache (3.5 % NEPA, 2.8 % oral palonosetron) and constipation (2.0 % NEPA, 2.2 % oral palonosetron). There were no serious treatment-related adverse events during cycle 1 or during the multiple-cycle extension for either treatment group. There were also no treatment-related adverse events leading to discontinuation and no deaths for NEPA-treated patients.
Cardiac safety
The percentage of patients with at least one adverse event classified as a cardiac disorder was similar for both groups in cycle 1 (2.6 % NEPA, 2.1 % oral palonosetron) and during the multicycle extension (5.0 % NEPA, 4.6 % oral palonosetron). The percent of patients with treatment-emergent ECG abnormalities was comparable between groups. The mean changes in QTcF were small and similar between groups and returned to baseline at 120 h. The percentage of patients with increases from baseline of >60 ms in QTcF were 0.7 and 1.1 % for NEPA and oral palonosetron, respectively, for cycle 1 and 1.5 and 2.6 % in the multiple-cycle extension. Similar proportions of patients had high troponin levels (i.e., >0.12 ng/mL) in cycle 1 (0.1 % NEPA vs 0.3 % oral palonosetron) and in the multicycle extension (3.4 % NEPA vs 2.9 % oral palonosetron). Of these, 0.4 % NEPA and 0.7 % oral palonosetron had troponin values greater than 0.50 ng/mL. In the majority of cases, the high values developed in cycles 5 and 6. Mean LVEF changes from screening to end of study were negligible and comparable between groups.
Discussion
The presence of CINV in the first cycle of chemotherapy has been established as a strong predictor of CINV in subsequent cycles [15, 16]. Therefore, the best available prophylactic antiemetics should be administered beginning at the first course of chemotherapy to maximize prevention of CINV from the start. All antiemetic guideline committees consistently suggest that combination regimens aimed at multiple molecular targets associated with emesis are now the standard of care for prevention of HEC and some types of MEC-induced CINV [1, 2].
Developed as the first antiemetic combination agent, NEPA presents a convenient approach to targeting what experts now believe are the two most important molecular targets (substance P/NK1 and serotonin/5-HT3) associated with CINV [6]. In this study, NEPA was administered with a single dose of dexamethasone. This offers a simple single-day “triplet” regimen and could reduce side effects associated with multiple-day dosing of dexamethasone.
This study evaluated 1450 patients over a total of almost 6000 chemotherapy cycles. While historically, the high dropout rate in multicycle trials has created methodologic challenges, in this study, 76 % of all patients completed at least 4 cycles of chemotherapy. The population selected included patients with solid tumors receiving an anthracycline-cyclophosphamide chemotherapy regimen. This combination has historically been considered a moderately emetogenic regimen but because it is commonly administered to (young) females with breast cancer (as was the case in this study), the emetogenic risk is substantially increased due to the additional patient-related risk factors [1, 2]. Consequently, the population evaluated in this study represents a group at significant risk for CINV. All antiemetic guidelines recommend NK1 RA/5-HT3RA/dexamethasone prophylaxis in this setting with guideline committees now classifying anthracycline-cyclophosphamide regimens as HEC. However, if an NK1 RA is not available, the Multinational Association for Supportive Care in Cancer (MASCC) recommends the use of palonosetron as the preferred 5-HT3 RA [2].
While this study was conducted in a relatively homogenous population of predominantly white females, other studies in the NEPA program have demonstrated efficacy and safety in a broader population of patients receiving various types of emetogenic chemotherapy [10, 11].
The data supporting the sustained efficacy benefit of NEPA over multiple cycles in this study is compelling for clinical practice. The NEPA benefit over oral palonosetron was maintained over multiple cycles for overall complete response rates and also for no emesis and no significant nausea in each cycle. These findings were consistent for all endpoints across the entire period of risk (0–120 h) for all four chemotherapy cycles.
Nausea remains a clinical challenge, and the nausea control rates were not as high as those for emesis. However, it is encouraging that treatment including NEPA resulted in a clinical benefit over oral palonosetron in preventing significant nausea over multiple cycles, as there have been no consistent findings to date supporting the role of other NK1 receptor antagonists in nausea control [17–21]. These results are supported by a prior phase 2 trial where NEPA resulted in superior nausea control over oral palonosetron in patients receiving highly emetogenic chemotherapy [10].
A complementary analysis offers perhaps a more rigorous approach to assessing preservation of efficacy in that it evaluates continued CR success over multiple cycles by censoring those patients who had emesis or more than mild nausea (i.e., CR failures); this approach was utilized previously in analyzing the sustained benefit of aprepitant over multiple cycles in a similar population of patients receiving AC chemotherapy [12]. In this study, the complementary analysis demonstrated significantly more NEPA-treated patients having continued CR success across cycles (log-rank test, p < 0.0001). It is notable that the incremental benefit of NEPA over oral palonosetron continued to increase across cycles with 13 % more NEPA-treated patients having continued CR by cycle 4. Similar results were seen for sustained nausea control (Fig. 4).
These findings are particularly impressive considering that NEPA (plus dexamethasone) was compared with and shown to be superior to oral palonosetron, the MASCC guideline-preferred 5-HT3 RA (plus dexamethasone) in the setting when an NK1 RA is unavailable. This suggests that the incremental benefit of the NEPA combination over an older 5-HT3 RA might be greater. Future trials could be considered to explore this as well as evaluating how NEPA would perform against an aprepitant-containing regimen. According to the data generated thus far, NEPA showed slightly higher response rates than an exploratory aprepitant arm in both a single-cycle study in patients receiving highly emetogenic chemotherapy [10] and over multiple cycles in patients receiving either non-AC moderately or highly emetogenic chemotherapy [13].
Consistent with the safety findings previously reported in cycle 1 of this study [13] and in the HEC/MEC multiple-cycle trial [11], NEPA was well tolerated over multiple cycles of chemotherapy without evidence for increasing adverse events over time/cycles or cardiac safety concerns for either agent. There continued to be a low incidence of treatment-related adverse events, none of which led to discontinuation, and there were no serious treatment-related adverse events or deaths for NEPA-treated patients.
Conclusion
The results of this study demonstrate superiority of NEPA over oral palonosetron (both given with dexamethasone) in preventing CINV and sustained efficacy over multiple cycles of chemotherapy. As a novel antiemetic combining a new NK1 RA with palonosetron (the guideline “preferred” 5-HT3 RA), NEPA offers promising efficacy with guideline-consistent antiemetic prophylaxis and may represent an advance in antiemetic treatment options.
References
Hesketh PJ, Bohlke K, Lyman GH et al (2016) Antiemetics: ASCO clinical practice focused guideline update. J Clin Oncol 34(4):381–386
Aapro M, et al. MASCC/ESMO Antiemetic Guideline, version 1.1 2016 - Multinational Association of Supportive Care in Cancer. Available at: http://www.mascc.org
Aapro M, Molassiotis A, Dicato M et al (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the pan European emesis registry (PEER. Ann Oncol 23(8):1986–1992
Gilmore JW, Peacock NW, Gu A et al (2014) Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract 10(1):68–74
Affronti ML, Schneider SM, Schlundt S et al (2014) Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer 22(7):1897–1905
Feyer P, Jordan K (2011) Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol 22(1):30–38
Rojas C, Slusher BS (2012) Pharmacological mechanism of 5-HT3 and tachykinin NK-1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 684(1–3):1–7
Stathis M, Pietra C, Rojas C, Slusher BS (2012) Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol 689(1–3):25–30
Thomas AG, Stathis M, Rojas C et al (2014) Netupitant and palonosetron trigger NK1 receptor internalization in NG 108-15 cells. Exp Brain Res 232(8):2637–2644
Hesketh PJ, Rossi G, Rizzi G et al (2014) Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 25:1340–1346
Gralla R, Bosnjak S, Hontsa A et al (2014) A phase 3 study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting (CINV) over repeated cycles of chemotherapy. Ann Oncol 25:1333–1339
Herrstedt J, Muss HB, Warr DG et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer 104(7):1548–1555
Aapro M, Rugo H, Rossi G et al (2014) A randomized phase 3 study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 25:1328–1333
Ewer M, Grunberg S, Ranganathan S et al (2009) Cardiac safety data for casopitant, an NK-1 receptor antagonist, given with anthracycline. Support Care Cancer 17:857–1039
Morrow G, Roscoe JA, Hickok JT et al (1998) Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology (Williston Park) 12:32–37
Cohen L, de Moor CA, Eisenberg P et al (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Rapoport BL, Jordan K, Boice JA et al (2010) Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chmotherapis and tumor types: a randomized, double-blind study. Supp Care Cancer 18(4):423–431
Warr DG, Hesketh PJ, Gralla RJ et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23(12):2822–2830
Poli-Bigelli S, Rodribues-Perelra J, Carides AD et al (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer 97(12):3090–3098
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the aprepitant protocol 052 study group. J Clin Oncol 21:4112–4119
Schmoll HJ, Aapro MS, Poli-Bigelli S et al (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17(6):1000–1006
Acknowledgments
The authors thank the clinical investigators, patients, and site personnel who participated in the study. We acknowledge the assistance of Jennifer Vanden Burgt, who provided medical writing support, liaised with authors, and coordinated the revisions, which was funded by Helsinn Healthcare. We also acknowledge Silvia Olivari and Silvia Sebastiani from Helsinn Healthcare and Kimia Kashef from Eisai, Inc. for critically reviewing the manuscript and members of the NEPA Publication Steering Committee (Dr. Paul Hesketh, Dr. Richard Gralla, and Dr. Karin Jordan) for their leadership and guidance. This work was supported by Helsinn Healthcare SA who provided the study drugs and the funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have the following conflicts of interest to disclose:
Aapro: consultant for Helsinn Healthcare, Eisai, Tesaro, and Merck; research funding from Helsinn Healthcare; speaker’s bureau for Helsinn Healthcare, Taiho, Eisai, Tesaro and Merck.
Karthaus: consultant for Helsinn Healthcare.
Schwartzberg: consultant for Helsinn Healthcare and Tesaro; research funding from Helsinn Healthcare.
Bondarenko: nothing to disclose.
Sarosiek: research funding from Helsinn Healthcare, Novartis, Parexel, Celltrion, Quintiles, Samsung, Lilly, Astra Zeneca, and GSK.
Oprean: consultant for Boehringer Ingelheim and MSD; honoraria received from Amgen, Sandoz, Astra Zeneca, Pfizer, and Lily.
Cardona-Huerta: travel/accommodations from Roche.
Hansen: nothing to disclose.
Rossi, Rizzi, and Borroni: employees of Helsinn Healthcare.
Rugo: research funding to University of California San Francisco from Eisai
Additional information
A prior publication reported the cycle 1 findings of this study [Aapro et al., Annals of Oncology 2014 NCT01339260]. This paper focuses on the findings in the multiple-cycle extension, data which was an oral presentation at both the ASCO and MASCC Annual Meetings in 2014.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aapro, M., Karthaus, M., Schwartzberg, L. et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Support Care Cancer 25, 1127–1135 (2017). https://doi.org/10.1007/s00520-016-3502-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3502-x