Abstract
Purpose
This study aims to determine the incidence of nausea and vomiting (CINV) after moderately emetogenic chemotherapy (MEC), under medical practice conditions and the accuracy with which physicians perceive CINV.
Methods
Chemotherapy-naive patients receiving MEC between April 2012 and May 2013 were included. Patients completed a diary of the intensity of nausea and number of vomiting episodes. Complete response and complete protection were assessed as secondary endpoints.
Results
Of 261 patients included, 240 were evaluated. Median age was 64 years, 44.2 % were female and 11.2 % were aged less than 50 years; 95.3 % of patients received a combination of 5-hydroxytryptamine 3 (5-HT3) antagonist + corticosteroid as antiemetic treatment. Vomiting within 5 days of chemotherapy administration occurred in 20.8 %, nausea in 42 % and significant nausea in 23.8 % of patients. An increase in the percentage of patients with significant nausea (from 9.4 to 21.7 %) and vomiting (from 9.2 to 16.5 %) was observed from the acute to the delayed phase. Complete response was 84.2 % in the acute phase, 77 % in the late phase and 68.9 % in overall period. Complete protection was 79.5 % in the acute phase, 68.8 % in the late phase and 62.4 % throughout the study period. Physicians estimated prophylaxis would be effective for 75 % of patients receiving MEC, compared with 54.1 % obtained from patients’ diary.
Conclusion
Despite receiving prophylactic treatment, 31 % of patients did not achieve a complete response and 38 % complete protection. In general, nausea was worse controlled than vomiting. The results also showed the late phase was worse controlled than the acute phase in all variables. Healthcare providers overestimated the effectiveness of antiemetic prophylaxis.
Similar content being viewed by others
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the most common and most feared adverse events that can be experienced by cancer patients [1–4]. CINV is associated with significant morbidity which has a negative effect on patient quality of life. Its occurrence essentially depends on the dose and type of chemotherapy agent used in treatment [5, 6].
There are currently many anti-emetic agents that may be used as prophylaxis and treatment for CINV, including 5-hydroxytryptamine 3 (5-HT3) receptor antagonists, corticosteroids, neurokinin 1 (NK-1) receptor antagonists, dopaminergic receptor antagonists, benzodiazepines, neuroleptics and cannabinoids [7]. However, despite the fact that antiemetic treatments have been improving over the years with the introduction of new drugs, there are still patients who are not protected against CINV [8, 9]. Nausea and delayed CINV (occurring >24 h post-chemotherapy) are reported as particular challenges in clinical practice [10–13].
Most studies that assess the incidence of chemotherapy-induced nausea and vomiting have been conducted in groups of patients who received highly emetogenic chemotherapy (HEC). There are very few studies conducted with moderately emetogenic chemotherapy (MEC), and these are mainly limited to anthracycline-cyclophosphamide (AC) treatments [13] which, nowadays, the majority of therapeutic guides consider to be HEC [5, 6]. For this reason, there is very little data related to the incidence of CINV in treatment-naive patients who receive MEC and collected systematically as for example by using a patient diary [14]. However, in studies that include nausea and vomiting as an objective, it is recommended that the information provided by the patients themselves is used, as this will allow for a greater ability to compare the regimens and data from various trials [4, 15]. Despite being able to accurately predict the prevalence of acute CINV, health professionals often underestimate the frequency of delayed nausea and vomiting, so closer monitoring is required by them throughout the complete treatment period [16].
There are hardly any studies in the medical literature with MEC regimen containing carboplatin and other regimens used in the treatment of colorectal cancer. The objective of this study was to determine the incidence of nausea and vomiting when appropriate antiemetic therapy is administered within various MEC regimens, ensuring that these regimens (carboplatin and oxaliplatin and/or irinotecan) were represented in the total sample, as well as to evaluate the perception Spanish physicians have regarding the incidence of CINV in these MEC regimens.
Patients and methods
Design
A prospective, observational multi-centre study conducted in 19 hospitals in Spain, between April 2012 and May 2013, in patients diagnosed with cancer who were chemotherapy-naive and who were scheduled to receive moderately emetogenic chemotherapy treatment (MEC). In order to be included in the study, patients had to have signed the informed consent form, be adults (≥18 years old) and be indicated for treatment with oxaliplatin and/or irinotecan in the case of colorectal cancer and with carboplatin or MEC regimens (no AC) in the case of any other type of cancer. Patients, who were unable to take oral medication, presented vomiting in the 24 h preceding the first chemotherapy cycle were under treatment with radiotherapy or presented with brain metastases were excluded from the study.
The allocation of a patient to a specific therapeutic strategy was not previously decided by the protocol but rather was determined by usual medical practice, and the decision to prescribe chemotherapy treatment was prior to patient inclusion.
The study was approved by the ethics committees at each of the participating sites and was conducted in accordance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki. All of the patients signed the informed consent.
Data collection and assessments
Investigators were instructed regarding the data to be recorded during the study. Before administering the MEC to patients, they had to answer three questions regarding their perception of the incidence of nausea and vomiting following chemotherapy administration, the use of rescue medication and their perception of the efficacy of the antiemetic measures prescribed.
Patients’ demographic data, clinical information on the cancer, metastases, chemotherapy regimen, administered prophylaxis for vomiting and/or nausea and antiemetic medication prescribed were recorded by the investigator during the course of the study. The study endpoints were complete response defined as no emesis and no use of rescue therapy for cycle 1 and complete protection defined as no emesis, no significant nausea and no use of rescue medication. Significant nausea was defined as nausea scored ≥25 mm to 100 mm visual analogue scale (VAS).
The patients who agreed to participate in the study received a diary covering the first 5 days following MEC administration. Patients were instructed on how to complete the diary, in which they had to record the following on a daily basis: vomiting and nausea episodes, the intensity of the nausea, determined through a horizontal 100 mm VAS of which the left end corresponded to “I have NO nausea today” and the right end to “the worst possible nausea”, as well as the use of antiemetic rescue medication. On the first day of the second cycle, they had to return the duly completed patient diary to the doctor.
Sample size
A maximum of approximately 285 subjects were recruited.
According to previous estimates of the percentage of patients with MEC who experience an episode of vomiting and assuming an accuracy of 10 %, 93 patients were needed to detect an incidence of vomiting of 40 %, with an overall significance of 0.05. Considering a 10 % of lost to follow up, the number of patients were 102 per group. Groups 2 and 3 included 90 patients each, and the analysis of these subgroups included also to patients in the overall MEC group meet the specific characteristics (or carboplatin-based regimens or patients diagnosed with CRC receiving oxaliplatin and/or irinotecan), a final sample of 102 patients in each group are guaranteed.
In the first phase, each site recruited seven patients treated with MEC regimens and then, other six patients subsequently were enrolled in each defined subgroup.
Statistical analysis
A descriptive analysis was performed on all of the variables (demographic variables and characteristics of the patients, the disease and the MEC treatment received) for all valid patients. Quantitative variables were described through their mean, median, standard deviation, minimum, maximum and the total number of patients with available data, and the qualitative variables were in the form of tables with relative and absolute frequencies. For the primary objective, the incidence of nausea and vomiting associated with MEC regimens was evaluated during the 5 days following administration, within the first 24 h following administration of the first chemotherapy cycle (acute phase) and during the four subsequent days (delayed phase), expressed using the percentage of patients with vomiting and the percentage of patients with nausea and their corresponding 95 % confidence intervals. For the secondary objectives, the percentage of patients with vomiting, nausea, significant nausea, use of rescue medication, lack of complete response and lack of complete protection were analysed for both acute and delayed phases of emesis.
The analyses performed with categorical result variables used the chi-square test or Fisher’s exact test. McNemar’s test was used for comparisons made between the two study phases (acute and delayed). The Cochran-Armitage trend test was used to evaluate a trend. The significance level used was 0.05. All of the statistical analyses were performed with SAS v.9.3 software.
Results
Patients
A total of 261 patients who received MEC were included in 16 hospitals between April 2012 and May 2013. Twenty-one of them were excluded from the main analysis due to not meeting one or more of the inclusion/exclusion criteria. Of the 240 patients analysed, 44.2 % were women and 11.2 % were under the age of 50, with the mean age of the population being 64.
The patients’ characteristics are shown in Table 1.
Colorectal cancer was the most common type of cancer, affecting 47.5 % of patients, followed by a lower incidence of lung cancer and ovarian cancer. The most frequent chemotherapy combinations were oxaliplatin + capecitabine in 25 % of cases and carboplatin + paclitaxel in 22.8 %. The antiemetic treatment of choice in 95.3 % of the patients was a 5-HT3 antagonist + corticosteroid (±another drug). If this is broken down by phase, in the acute phase, the most common prophylactic treatment was the combination of setron + corticosteroid in 94.9 % followed by metoclopramide + corticosteroid in 4.7 % of patients and one patient did not receive prophylaxis; and in the delayed phase, the distribution was 56.2 % did not receive prophylaxis in this phase, the most used prophylactic treatment was metoclopramide + corticosteroid in 25.5 % followed by setron + corticosteroid in 17 % and setron alone in 1.3 % of patients.
Incidence of chemotherapy-induced nausea and vomiting (CINV)
Despite the use of antiemetic prophylaxis, a total of 103 patients (44.6 %) presented with nausea and/or vomiting at some point during the 120 h following administration of chemotherapy. Approximately 20.8 % experienced at least one episode of vomiting, 42 % nausea of any intensity and significant nausea in 23.8 % of patients.
In relation to CINV, no significant differences were found with risk factors, age, sex and alcohol consumption except for history of kinetosis (p = 0.0281; chi-quare test).
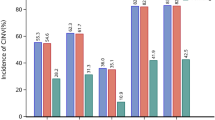
Both the incidence of nausea and vomiting were much more frequent in the delayed phase than during the acute phase. This increase was statistically significant for all categories (vomiting, nausea and significant nausea) and increased from 9.2 to 16.5 % (p = 0.0112; McNemar’s test), from 23.3 to 38.5 % (p < 0.0001; McNemar’s test) and from 9.4 to 21.7 % (p = 0.0002; McNemar’s test), respectively. These data are shown in Fig. 1. The analysis by treatment group did not show significant differences between the different chemotherapy regimens administered (Table 2). Particularly, the overall incidence of nausea and vomiting in patients received XELOX regimens and patients received other regimens did not reach statistical significance (42.9 vs. 45.3 %; p = 0.7270; chi-square test).
Complete response and complete protection
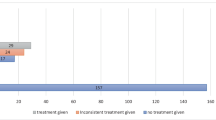
Approximately three out of every ten patients (31.1 %) did not achieve a complete response, and four out of every ten patients (37.6 %) did not reach complete protection in spite of the prophylactic treatment administered (Fig. 2). As it can be seen in Table 3, the lack of complete response was higher during the delayed phase than that observed in the acute phase (23 vs. 15.8 %) and a similar situation was observed on evaluating the complete protection of the patients; 31.2 % presented a lack of complete protection in the delayed phase compared to 20.5 % of patients in the acute phase.
Of the total number of patients, 17.9 % required rescue medication compared to 82.1 % who did not need it. Approximately 7.2 % of patients required it during the acute phase and double this number (14.5 %) in the delayed phase. By treatment group, the frequency of use of rescue medication was lower in the patients who received oxaliplatin and/or irinotecan, although the differences were not statistically significant. The rescue medication prescribed was metoclopramide in 26.4 % of patients and ondansetron in 12.3 %.
Only 22 of the 229 patients who received a second MEC cycle had their antiemetic treatment changed. Ten of these patients had a lack of complete response or complete protection with the prophylaxis administered in the first cycle.
Physicians’ perception
Figure 3 shows the differences observed between the incidence of CINV predicted by the investigators and that observed in the study. According to the investigators, the expected percentage of patients with nausea and/or vomiting was 10 % lower than the data observed in the study (35 vs. 45 %) and, in addition, they believed that the prophylaxis antiemetic treatment would be effective (no nausea, no vomiting and no use of rescue medication) for 75 % of the patients who received MEC, while the actual rate observed in the study was for 54 %, which means a difference of 21 percentage points between the efficacy predicted by the investigators and reality. On the other hand, the prediction by investigators of rescue medication use and that actually used in the study was quite accurate.
Discussion
Chemotherapy-induced nausea and vomiting are a serious problem for cancer patients and have a great impact on their quality of life. Although preventive guidelines and the development of new antiemetic agents have considerably reduced the incidence of CINV, between 13 and 32 % of patients who receive HEC or MEC chemotherapy experience vomiting or the need for rescue medication during the acute phase and more than 35 % present with nausea. However, the incidence of CINV in the delayed phase increases up to 52 % for nausea and 28 % for vomiting [16, 17] in patients treated with MEC and antiemetic prophylaxis in regular medical practice.
Our study is the only multi-centre study that has determined the incidence of CINV in Spain under routine clinical practice conditions. The results indicate a rate of 42 % for nausea and 21 % for vomiting in the first 5 days following chemotherapy administration. The differences observed between the acute and the delayed phases concur with data from other studies [16, 18, 19]. Approximately 9.2 % of patients experienced vomiting in the acute phase of the first cycle and 16.4 % in the delayed phase, with the incidence of nausea being higher, 23.3 and 38.5 %, respectively, in the two phases. This indicates that there is poorer control of chemotherapy effects in the days following administration. Similar results have recently been published with regard to the Asian population in patients treated with MEC regimens [20], and these also highlight poorer control of CINV in the delayed phase compared to the acute phase for the entire population. There were no significant differences between the populations in the various countries in the study during the acute phase, but there were in the data for the delayed phase.
The objective of antiemetic treatment should be complete prevention of chemotherapy-induced nausea and vomiting for the majority of patients who receive chemotherapy treatment. The frequency of nausea and vomiting depends primarily on the chemotherapy agent used, in addition to other factors such as age, gender, alcohol consumption and history of kinetosis (Figure S1). In our study, a significant upward trend was observed between the number of factors present and a greater incidence of CINV. However, unlike in other studies [13], no significant association was found with any of the factors alone, except for history of kinetosis.
Nevertheless, the poor control of nausea and vomiting in the acute phase turned out to be an important risk factor for the lack of control of these events in the delayed phase [21]. These symptoms may appear, however, without acute nausea or emesis [16]. In addition, the patients who presented with delayed nausea and vomiting in the first cycle of chemotherapy have a greater risk of presenting with these symptoms in subsequent cycles [22]; therefore, good control of nausea and vomiting in the first cycle is necessary in order to control them well in subsequent chemotherapy cycles.
It is remarkable how, despite having the improved prevention and control of acute emesis, the same has not occurred in the delayed phase, and this continues to be a significant problem in patients who receive chemotherapy, particularly in terms of nausea.
Current guidelines [23, 24] recommend that management of CINV should be based primarily on the emetogenic potential of the drugs selected for chemotherapy, which have been classified into four emetogenic risk groups: high, moderate, low and minimal. Nowadays, for patients who receive regimens with a high emetogenic potential, the combination of 5-HT3 receptor antagonist, aprepitant and dexamethasone is recommended before chemotherapy, while in regimens with moderate emetogenic potential, the guidelines establishes the use of palonosetron + dexamethasone, but the key question is whether aprepitant should be part of antiemetic prophylaxis as a third agent [25]. In this context, all guindelines (ASCO; MASCC/ESMO and NCCN) suggest that the triple combination (aprepitant, 5-HT3 receptor antagonist and dexamethasone) should be used only in patients who receive AC regimens [9].
Antiemetic medication is also recommended in the days following chemotherapy in order to prevent delayed vomiting and nausea [25]. The adherence to the guidelines and its application in the management of patients treated with chemotherapy regimens with high and moderate emetogenic potential enables the incidence of CINV to be reduced and may mean that 10 % more patients achieve a complete response [22]. However, compliance with these guidelines appears to be very low and the prescription of antiemetics to patients with HEC or MEC regimens is often inadequate. There is a good deal of variation with regard to doses administered and suboptimal control of CINV [8, 26], particularly in the days following the chemotherapy when patients are at home and not under the direct supervision of clinic staff [26, 27]. This observation is in line with those made by Doranne [13]; while the patients who developed CINV on the day of their treatment were likely to receive additional antiemetic treatment, the symptoms they developed after 2 to 5 days did not affect CINV management, as demonstrated by the data from the study.
In our study, the prophylaxis treatment of choice in 95.3 % of the patients was 5-HT3 + corticosteroid, and rescue medication was prescribed to 37 % of patients. In spite of this, 31.1 % of patients did not achieve complete response and 37.6 % presented with vomiting or significant nausea or required rescue medication, this being more significant in the delayed phase than in the acute phase. As shown in our results, prophylactic antiemetic treatment always included an corticosteroid, both in the acute phase and the late phase, evidencing the importance of adherence to corticosteroids for the enhancing the antiemetic effect of metoclopramide and 5-HT3 antagonists.
However, our results confirm that physicians’ perception of the incidence of CINV in MEC regimens is not in line with reality [28], since they underestimate said rate [16, 29]. In our case, investigators underestimated the incidence of nausea and vomiting for the overall population and regimens with carboplatin. However, they overestimated CINV in patients with colorectal cancer who received oxaliplatin and/or irinotecan regimens, and the efficacy of the antiemetic treatment. A total of 52 % of patients did not make use of the rescue medication prescribed. The underestimation of the incidence of delayed CINV leads to undertreatment of delayed emesis. It seems clear that despite the existence of guidelines and protocols for treatment and follow-up of patients receiving MEC, the adherence to them in the clinical practice is very low. Communication between professional and patients about this side effect may help improve outcomes. Addressing misconceptions and establishing mutually consistent goals will lead to more effective overall care. It is necessary to establish and to improve efficient education and training of clinicians/nurses. The use of standardized protocols that include chemotherapy antiemetic treatment required according to the emetogenic risk, as well as the monitoring of patients receiving MEC by use of “patient diary”.
The primary limitations of this study, on one hand, consist of the consequences of its observational design and the lack of follow-up until several cycles had been completed. And on the other, we cannot rule out errors or omissions made when patients completed the diary.
In conclusion, the results of this study reveal that the patients who receive moderately emetogenic chemotherapy continue to experience nausea and vomiting despite the antiemetic treatment administered. Furthermore, the advances achieved in controlling vomiting in the acute phase do not correspond to better control in the delayed phase, where nausea is still not effectively controlled.
Finally, we believe it is necessary to implement actions that ensure the knowledge and application of the antiemetic guidelines or recommendations agreed upon by various expert organisations when establishing prophylactic antiemetic measures and to show that, in the majority of cases, physicians underestimate the incidence of nausea and vomiting in patients receiving MEC.
References
Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, Tattersall MH (1983) On the receiving end patient perception of the side effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19:203–208
de Boer-Dennert M, de Wit R, Schmitz PIM, Djontono J, van Beurden V, Stoter G, Verweij J (1997) Patient perception of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Passik SD, Kirsh KL, Rosenfeld B, McDonald MV, Theobald DE (2001) The changeable nature of patients’ fear regarding chemotherapy: implications for palliative care. J Pain Symptom Manag 21:113–120
Wagland R, Richardson A, Armes J, Hankins M, Lennan E, Griffiths P (2014) Treatment-related problems experienced by cancer patients undergoing chemotherapy: a scoping review Eur J Cancer Care. doi: 10.1111/ecc.12246
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR et al (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E et al (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(suppl 5):v232–v243. doi:10.1093/annonc/mdq194
Feyer P, Jordan K (2011) Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol 22:30–8
Hesketh PJ (2008) Drug therapy: chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494
Jordan K, Gralla R, Jahn F, Molassiotis A (2014) International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol 722:197–202
Roscoe JA, Morrow GR, Hickok JT, Stern RM (2000) Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manag 20:113–121
Perwitasari DA, Atthobari J, Mustofa M, Dwiprahasto I, Hakimi M, Gelderblom H, Putter H, Nortier JW, Guchelaar HJ, Kaptein AA (2012) Impact of chemotherapy-induced nausea and vomiting on quality of life in Indonesian patients with gynecologic cancer. Int J Gynecol Cancer 22:139–45
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK (2012) Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 20:107–117
Diario y Agenda Personal de Salud. Sociedad Española de Oncología Médica (SEOM) y Sociedad Española de Enfermería Oncológica (SEEO) 2008. http://seom.org/seomcms/images/stories/recursos/sociosyprofs/documentacion/boletinseom/2010/69/14publicaciones.seom.bol69.pdf
Basch E, Snyder C, McNiff K, Brown R, Maddux S, Smith ML, Atkinson TM, Howell D, Chiang A, Wood W, Levitan N, Wu AW, Krzyzanowska M (2014) Patient-reported outcome performance measures in oncology. J Oncol Pract 10:209–211
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G et al (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268
Gómez-Raposo C, Feliú-Batle J, González-Barón M (2006) Prevención y control de las náuseas y vómitos inducidos por quimioterapia. Med Clin 126:143–51
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F, On behalf of the Aprepitant Protocol 054 Study Group (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer 97:3090–3098. doi:10.1002/cncr.11433
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ, Aprepitant Protocol 052 Study Group (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin. J Clin Oncol 21:4112–9
Hsieh RK, Chan A, Kim HK, Yu S, Kim JG, Lee MA, Dalén J, Jung H, Liu YP, Burke TA, Keefe DM (2015) Baseline patient characteristics, incidence of CINV, and physician perception of CINV incidence following moderately and highly emetogenic chemotherapy in Asia Pacific countries. Support Care Cancer 23:263–72
Schnell FM (2003) Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist 8:187–98
Aapro M, Molassiotis A, Dicato M, Peláez I, Rodríguez-Lescure Á, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F, on behalf of the PEER investigators (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER). Ann Oncol 23:1986–1992
Antiemesis. NCCN practice guidelines in oncology v2 2014. National Comprehensive Cancer Network www.nccn.org. Last accessed September 4, 2014
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM et al (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–47
Navari RM (2007) Overview of the updated antiemetic guidelines for chemotherapy-induced nausea and vomiting. Commun Oncol 4:3–11
De Angelis V, Roila F, Sabbatini R, Italian Group for Antiemetic Research et al (2003) Cancer chemotherapy (CT)-induced delayed emesis: antiemetic prescriptions in clinical practice (abstract 2971). J Clin Oncol 22:739
Rubenstein EB, Litke SE, Jose MA (2002) Chemotherapy-induced nausea and emesis in the 5HT3 era: a national survey of hematologists/oncologists [abstract]. Proc Am Soc Clin Oncol 21:260b
Majem M, Moreno ME, Calvo N, Feliu A, Pérez J, Mangues MA, Barnadas A (2011) Perception of healthcare providers versus patient reported incidence of chemotherapy-induced nausea and vomiting after the addition of NK-1 receptor antagonists. Support Cancer Care 19:1983–90. doi:10.1007/s00520-010-1042-3
Kris MG (2003) Why do we need another antiemetic? Just Ask. J Clin Oncol 21:4077–80
Conflict of interest
All authors have declared no conflicts of interest. The research has been funded by Merck Sharp & Dohme (MSD). Merck Sharp & Dohme Spain—a subsidiary of Merck & Co. Inc., Whitehouse Station, New Jersey, USA—provided Financial Support for the conduct of the study.
Author’s contributions
All authors have contributed to the research, they had access to all data, have read and approved the text and are responsible for sending to Support Cancer Care for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yolanda Escobar and Gerardo Cajaraville contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOCX 189 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Escobar, Y., Cajaraville, G., Virizuela, J.A. et al. Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer 23, 2833–2840 (2015). https://doi.org/10.1007/s00520-015-2809-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2809-3