Abstract
Background
The learning curve for robotic colorectal surgery is ill-defined. This study aimed to investigate the learning curve of experienced laparoscopic rectal surgeons when starting with robotic total mesorectal excision (TME) using cumulative sum (CUSUM) charts.
Methods
This retrospective case series analysed patients who underwent curative and elective laparoscopic or robotic TMEs for rectal cancer performed by two surgeons. The first consecutive robotic TME cases of each surgeon were 1:1 propensity score matched to their laparoscopic TME cases using age, body mass index, American Society of Anesthesiologists grade, T stage (AJCC) and tumour location height. The matched laparoscopic cases defined individual standards for the quality indicators: operating time, R stage, lymph node harvest, length of hospital stay and major complications (Clavien–Dindo grade 3–5). Deviation of more than a quarter of a standard deviation from the mean for the continuous indicators, or exceeding the observed risk for the binary indicators was defined as off-target with an upward inflection in the CUSUM curve.
Results
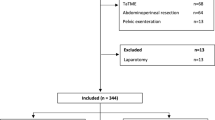
From 2006 to 2015, 384 (294 laparoscopic; 90 robotic) TMEs met the inclusion criteria. Surgeon A performed 206 (70.1%) of the laparoscopic and 43 (47.8%) of the robotic cases. Surgeon B performed 88 (29.9%) of the laparoscopic and 47 (52.2%) of the robotic cases. After matching, no covariate exhibited an absolute standardised mean difference >0.25. For surgeon A, the CUSUM curves showed no apparent learning process compared to his laparoscopic standards. For surgeon B, a learning process for operation time, lymph node harvest and major complications was demonstrated by an initial upward inflection of the CUSUM curves; after 15 cases, all quality indicators were generally on target.
Conclusions
For experienced laparoscopic colorectal surgeons, the formal learning process for robotic TME may be short to reach a similar performance level as obtained in conventional laparoscopy.


Similar content being viewed by others
References
Shah J, Vyas A, Vyas D (2014) The History of Robotics in Surgical Specialties. Am J Robot Surg 1:12–20
Feroci F, Vannucchi A, Bianchi PP, Cantafio S, Garzi A, Formisano G, Scatizzi M (2016) Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J Gastroenterol 22:3602–3610
D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G (2013) Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 27:1887–1895
Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B (2010) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 24:2888–2894
Araujo SE, Seid VE, Klajner S (2014) Robotic surgery for rectal cancer: current immediate clinical and oncological outcomes. World J Gastroenterol 20:14359–14370
Wang M, Kang X, Wang H, Guan W (2014) A meta-analysis on the outcomes and potential benefits of hybrid robotic technique in rectal cancer surgery. Zhonghua Wei Chang Wai Ke Za Zhi 17:785–790
Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gulla N, Noya G, Boselli C (2012) Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 14:e134–e156
Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, Garbett C, Guillou P, Holloway I, Howard H, Marshall H, McCabe C, Pavitt S, Quirke P, Rivers CS, Brown JM (2012) An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 27:233–241
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29:1679–1685
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Ahmed J, Kuzu MA, Figueiredo N, Khan J, Parvaiz A (2016) Three-step standardized approach for complete mobilization of the splenic flexure during robotic rectal cancer surgery. Colorectal Dis 18:O171–O174
Bolsin S, Colson M (2000) The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care 12:433–438
Chang WR, McLean IP (2006) CUSUM: a tool for early feedback about performance? BMC Med Res Methodol 6:8
Kestin IG (1995) A statistical approach to measuring the competence of anaesthetic trainees at practical procedures. Br J Anaesth 75:805–809
Montgomery DC (2009) Introduction to statistical quality control, 6th edn. Wiley, Hoboken
Thoemmes F (2012) Propensity score matching in SPSS. https://arxiv.org/ftp/arxiv/papers/1201/1201.6385.pdf. asXiv:1201.6385; Accessed 18 Augu 2016.
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ho DE, Imai K, King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:28
Bowers J, Fredrickson M, Hansen B (2010) RItools: Randomization Inference Tools. pp R package
Iacus S, King G, Porro G (2009) cem: Sorftware for Coarsened Exact Matching. pp R package
Colquhoun PH (2008) CUSUM analysis of J-pouch surgery reflects no learning curve after board certification. Can J Surg 51:296–299
Biau DJ, Resche-Rigon M, Godiris-Petit G, Nizard RS, Porcher R (2007) Quality control of surgical and interventional procedures: a review of the CUSUM. Qual Saf Health Care 16:203–207
Bowles TA, Watters DA (2007) Time to CUSUM: simplified reporting of outcomes in colorectal surgery. ANZ J Surg 77:587–591
Trinh BB, Jackson NR, Hauch AT, Hu T, Kandil E (2014) Robotic versus laparoscopic colorectal surgery. JSLS 18:3
Fung AK, Aly EH (2013) Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum 56:786–796
de Jesus JP, Valadao M, de Castro Araujo RO, Cesar D, Linhares E, Iglesias AC (2016) The circumferential resection margins status: A comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol 42:808–812
Baxter NN (2009) Is lymph node count an ideal quality indicator for cancer care? J Surg Oncol 99:265–268
Wang J, Kulaylat M, Rockette H, Hassett J, Rajput A, Dunn KB, Dayton M (2009) Should total number of lymph nodes be used as a quality of care measure for stage III colon cancer? Ann Surg 249:559–563
Cooper MA, Ibrahim A, Lyu H, Makary MA (2015) Underreporting of robotic surgery complications. J Healthc Qual 37:133–138
Scarpinata R, Aly EH (2013) Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum 56:253–262
Acknowledgements
The authors thank Sarah Marley, M.Sc., for her support in reviewing and providing statistical advice on this manuscript. Special thanks to Karen Flashman for data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Manfred Odermatt, Jamil Ahmed, Sofoklis Panteleimonitis, Jim Khan and Amjad Parvaiz have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Odermatt, M., Ahmed, J., Panteleimonitis, S. et al. Prior experience in laparoscopic rectal surgery can minimise the learning curve for robotic rectal resections: a cumulative sum analysis. Surg Endosc 31, 4067–4076 (2017). https://doi.org/10.1007/s00464-017-5453-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5453-9