Abstract
Purpose
In order to confirm the efficacy, tolerability, and baseline prognostic factors of an epirubicin (EPR)-containing triplet regimen, the EOF5 regimen, in patients with metastatic gastric cancer (MGC), we conducted the phase II trial and retrospective analysis.
Methods
MGC patients received the EOF5 regimen (EPR 50 mg/m2 and oxaliplatin (OX) 130 mg/m2 on day 1 followed by continuous infusion of 5-fluorouracil (5-FU) 375–425 mg/m2/days for 5 days every 3 weeks). Log-rank tests were used for univariate analysis of time to progression (TTP) and overall survival rate (OS), and stepwise Cox proportional hazards regression modeling was performed to generate a prognostic index.
Results
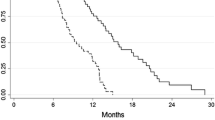
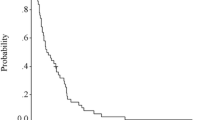
A total of 158 patients received the EOF5 regimen. Of the 150 evaluable patients, complete remission, partial remission, and stable disease were observed in 5 (3.3 %), 70 (46.7 %), and 58 patients (38.7 %), respectively. The median TTP and OS were 6.0 (95 % CI 5.4–6.6) and 12.6 months (95 % CI 8.2–16.9), respectively. Grade 3–4 neutropenia (44.0 %), thrombocytopenia (25.3 %), and anemia (6.7 %) were recorded. A prognostic index that included liver and lung metastasis, ascites/pleural effusion, and baseline serum CA19-9 was used to categorize the patients into three groups: good risk (0 risk factors), moderate risk (1 or 2 risk factors), and poor risk (3 or 4 risk factors). The median OS for these groups was 30.4, 12.4, and 5.6 months, respectively (P < 0.001).
Conclusions
EOF5 is an effective regimen and a suitable alternative for the first-line treatment of MGC. According to the prognostic index used in our study, patients with no risk factors have a better OS when treated with EOF5 than those with one or more risk factors.



Similar content being viewed by others
Abbreviations
- CAP:
-
Capecitabine
- CDDP:
-
Cisplatin
- CTC:
-
National Cancer Institute Common Toxicity Criteria
- DCF:
-
Combination regimen of docetaxel, CDDP, and 5-FU
- ECF:
-
Combination regimen of EPR, CDDP, and 21-day continuous infusion of 5-FU
- EOF5:
-
Combination regimen of EPR, OX, and 5-day continuous infusion of 5-FU
- EPR:
-
Epirubicin
- MGC:
-
Metastatic gastric cancer
- OX:
-
Oxaliplatin
- LN:
-
Lymph node
- SFDA:
-
State Food and Drug Administration of China
References
Ajani JA (2006) Standard of care for gastric cancer based on meta-analysis? Treading on thin ice or it is very nice! J Clin Oncol 24:5473–5474
Al-Batran SE, Jäger E, Scholz M (2007) Chemotherapy for advanced gastric cancer. J Clin Oncol 25:729
Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ (2004) Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 22:2395–2403
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Giessen C, Laubender RP, Fischer von Weikersthal L, Schalhorn A, Modest DP, Stintzing S, Haas M, Mansmann UR, Heinemann V (2013) Early tumor shrinkage in metastatic colorectal cancer: retrospective analysis from an irinotecan-based randomized first-line trial. Cancer Sci 104:718–724
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Kang JH, Lee SI, Lim do H, Park KW, OH SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30:1513–1518
Kim JG, Ryoo BY, Park YH, Kim BS, Kim TY, Im YH, Kang YK (2008) Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol 61:301–307
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Koo DH, Ryu MH, Ryoo BY, Lee SS, Moon JH, Chang HM, Lee JL, Kim TW, Kang YK (2012) Three-week combination chemotherapy with S-1 and cisplatin as first-line treatment in patients with advanced gastric cancer: a retrospective study with 159 patients. Gastric Cancer 15:305–312
Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kang HJ, Kim WK, Lee JS, Kang YK (2007) Combination chemotherapy with capecitabine (X) and Cisplatin (P) as first line treatment in advanced gastric cancer: experience of 223 patients with prognostic factor analysis. Jpn J Clin Oncol 37:30–37
Mansmann UR, Sartorius U, Laubender RP, Giessen CA, Esser R, Heinemann V (2012) Deepness of response: a quantitative analysis of its impact on post-progression survival time after first-line treatment in patients with mCRC. J Clin Oncol 30(suppl 34; abstr 427)
Mansmann UR, Sartorius U, Laubender RP, Giessen CA, Esser R, Heinemann V (2013) Quantitative analysis of the impact of deepness of response on post-progression survival time following first-line treatment in patients with mCRC. J Clin Oncol 31(suppl; abstr 3630)
Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T, Lofts F, Norman A (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Roth AD (2003) Curative treatment of gastric cancer: towards a multidisciplinary approach? Crit Rev Oncol Hematol 46:59–100
Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y (2014) Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 17:26–33
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O’Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15:261–267
Zhu X, Leaw J, Gu W, Qian Y, Du H, Wang B, Hong X, Yin J (2008) Phase II clinical trial of advanced and metastatic gastric cancer based on continuous infusion of 5-fluorouracil combined with epirubicin and oxaliplatin. J Cancer Res Clin Oncol 134:929–936
Acknowledgments
We thank Wenyuan Zhu, Yan Yan, Xuedan Sheng, and Hui Sun for their assistance in conducting this trial. We also thank Ms. Xiaofeng Gu for her documentation of the study data and secretarial assistance. Project partially supported by the Natural Science Foundation of Shanghai (Grant No. 13ZR1408200).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov ID: NCT00767377.
Rights and permissions
About this article
Cite this article
Zhu, X., Zhao, X., Peng, W. et al. Epirubicin combined with oxaliplatin and 5-day continuous infusion of 5-fluorouracil as a first-line treatment for metastatic gastric cancer: treatment outcomes and analysis of prognostic factors. J Cancer Res Clin Oncol 141, 109–118 (2015). https://doi.org/10.1007/s00432-014-1754-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1754-8