Abstract
Purpose
This study was performed to examine possible use of thymidine kinase 1 concentration in serum (STK1) for prognosis of non-Hodgkin’s lymphoma patients following chemotherapy treatment.
Methods
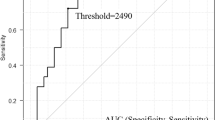
The STK1 levels of 37 patients were determined by enhanced chemiluminescent dot-blot assay on the day before chemotherapy, and on day 1 and day 28 after start of the treatment. The specificity and sensitivity was evaluated by Western blot with anti-TK1 IgY antibody and by receiver operating characteristic (ROC) analysis.
Results
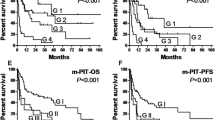
Western blot and ROC analysis of TK1 in serum showed high specificity and sensitivity. The mean STK1 level of the non-Hodgkin’s lymphoma patients was significantly higher compared to healthy persons (p < 0.001). The mean STK1 level increased significantly (p < 0.001) on day 1 and then declined, reaching on day 28 values corresponding to those of healthy persons. The mean STK1 values before treatment and at 1 and 28 days after start of the treatment also correlated significantly with the clinical response (CR, PR and NR) and five-year survival.
Conclusion
Although the number of patients was limited in this study, TK1 in serum might possess an important reference value in the evaluation of treatment and prognosis of non-Hodgkin’s lymphoma following chemotherapy.





Similar content being viewed by others
References
Ferguson RE, Carroll HP, Harris E, Maher ER, Selby PJ, Banks RE (2005) Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics 5:566–577
Gronowitz JS, Hagberg H, Kallander CFR, Simonsson B (1983) The use of serum deoxythymidine kinase as a prognostic marker and in the monitoring of patients with non-Hodgkin’s lymphoma. Br J Cancer 47:487–495
He Q, Wang N, Skog S, Eriksson S, Tribukait B (1996) Characterization of a peptide antibody against a C-terminal part of human and mouse cytosolic thymidine kinase, which is a marker for cell proliferation. Eur J Cell Biol 70:117–124
He E, Mao Y, Wu J et al (2004) Cytosolic thymidine kinase is a specific histopathologic tumour marker for breast carcinomas. Int J Oncol 25:945–953
He Q, Zhang P, Zou L et al (2005) Concentration of thymidine kinase 1 in serum (S-TK1) is a more sensitive proliferation marker in human solid tumours than its activity. Oncol Rep 14:1013–1019
HengZhi C, Zhou H, Tian NB, He E, Skog S (2008) Serological thymidine kinase 1 (STK1) indicates an elevated risk for development of malignant tumors. Anticancer Res 28:3897–3908
Kauffman MG, Kelly TJ (1991) Cell cycle regulation of thymidine kinase: residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol Cell Biol 11:2538–2546
Ke PY, Chang CF (2004) Mitotic degradation of human thymidine kinase 1 is dependent on the anaphase-promoting complex/cyclosome-CDH1-mediated pathway. Mol Cell Biol 24:514–526
Kok M, Bonfrer JM, Korse CM, de Jong D, Kersten MJ (2003) Serum soluble CD27, but not thymidine kinase, is an independent prognostic factor for outcome in indolent non-Hodgkin’s lymphoma. Tumour Biol 24:53–60
Poley S, Stieber P, Nussler V et al (1997) Serum thymidine kinase in non-Hodgkin lymphomas with special regard to multiple myeloma. Anticancer Res 17:3025–3029
Rehn S, Glimelius B, Sundstrom CA (1991) Comparative study of proliferation-associated parameters in B-cell non-Hodgkin’s lymphoma. Hematol Oncol 9:287–298
Sadamori N, Ichiba M, Mine M et al (1995) Clinical significance of serum thymidine kinase in adult T-cell leukaemia and acute myeloid leukaemia. Br J Haematol 90:100–105
Suki S, Swan F, Tucker S et al (1995) Risk classification for large cell lymphoma using lactate dehydrogenase, beta-2 microglobulin, and thymidine kinase. Leuk Lymph 18:87–92
Topolcan O, Lubos Holubec L (2008) The role of thymidine kinase in cancer diseases. Expert Opin Med Diagn 2:129–140
Törnevik Y, Ullman BJ, Balzarini J et al (1995) Cytotoxicity of 3′-azido-3′-deoxythymidine correlates with 3′-azidothymidine-5′-monophosphate (AZTMP) levels, whereas anti-human Immunodeficiency virus (HIV) activity correlates with 3′-azidothymidine 5′-triphosphate (AZTTP) levels in cultured CEM T-lymphoblastoid cells. Biochem Pharmacol 49:829–837
Wu CJ, Yang RJ, Zhou J et al (2003) Production and characterisation of a novel chicken IgY antibody raised against C-terminal peptide from human thymidine kinase 1. J Immunol Methods 277:157–169
Xu XH, Zhang YM, Shu XH et al (2008) Serological thymidine kinase 1 reflects progression of pre-malignant and malignant tumours during therapy. Mol Med Rep 1:705–711
Zhang ZN, Shen D (1998) Diagnosis of blood disease and response criteria. The second version. Beijing Science Press, Beijing, pp 168–349
Zhang F, Li H, Pendleton AR et al (2001) Thymidine kinase 1 immunoassay: a potential marker for breast cancer. Cancer Detect Prev 25:8–15
Zhang J, Jia Q, Zou S et al (2006) Thymidine kinase 1: a proliferation marker for determining prognosis and monitoring the surgical outcome of primary bladder carcinoma patients. Oncol Rep 15:455–461
Zou L, Zhang PG, Zou S, Li Y, He Q (2002) The half-life of cytosolic thymidine kinase in serum by ECL dot bolt: a potential marker for monitoring the response to surgery of patients with gastric cancer. Int J Biol Marker 17:135–140
Acknowledgments
This investigation was supported by Sino-Swed Tongkang Bio-Tech Ltd., Shenzhen, China, and by Departments No. 85 and No. 411 at PLA Hospital, Shanghai, China.
Conflict of interest statement
Zhu-Lin Pan, Xing-Ying Ji and Yan-Min Shi report no conflict of interest. Ji Zhou is CEO of SSTK Ltd, and Ellen He and Sven Skog are scientific consulters to SSTK Ltd., the company that produces the TK1 kit. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pan, ZL., Ji, XY., Shi, YM. et al. Serum thymidine kinase 1 concentration as a prognostic factor of chemotherapy-treated non-Hodgkin’s lymphoma patients. J Cancer Res Clin Oncol 136, 1193–1199 (2010). https://doi.org/10.1007/s00432-010-0769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0769-z