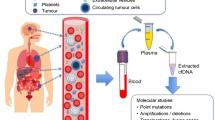
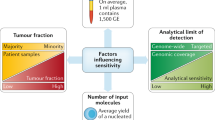
Abstract
Recently, the advent of therapies targeting genomic alterations has improved the care of patients with certain types of cancer. While molecular targets were initially detected in nucleic acid samples extracted from tumor tissue, detection of nucleic acids in circulating blood has allowed the development of what has become known as liquid biopsies, which provide a complementary and alternative sample source allowing identification of genomic alterations that might be addressed by targeted therapy. Consequently, liquid biopsies might rapidly revolutionize oncology practice in allowing administration of more effective treatments. Liquid biopsies also provide an approach towards short-term monitoring of metastatic cancer patients to evaluate efficacy of treatment and/or early detection of secondary mutations responsible for resistance to treatment. In this context, pathologists, who have already been required in recent years to take interest in the domain of molecular pathology of cancer, now face new challenges. The attitude of pathologists to and level of involvement in the practice of liquid biopsies, including mastering the methods employed in molecular analysis of blood samples, need close attention. Regardless of the level of involvement of pathologists in this new field, it is mandatory that oncologists, biologists, geneticists, and pathologists work together to coordinate the pre-analytical, analytical, and post-analytical phases of molecular assessment of tissue and liquid samples of individual cancer patients. The challenges include (1) implementation of effective and efficient procedures for reception and analysis of liquid and tissue samples for histopathological and molecular evaluation and (2) assuring short turn-around times to facilitate rapid optimization of individual patient treatment. In this paper, we will review the following: (1) recent data concerning the concept of liquid biopsies in oncology and its development for patient care, (2) advantages and limitations of molecular analyses performed on blood samples compared to those performed on tissue samples, and (3) short-term challenges facing pathologists in dealing with liquid biopsies of cancer patients and new strategies to early detect metastatic tumor cell clones.
Similar content being viewed by others
References
Committee on Policy Issues in the Clinical Development and Use of Biomarkers for Molecularly Targeted Therapies, Board on Health Care Services, Institute of Medicine, The National Academies of Sciences, Engineering, and Medicine; Graig LA, Phillips, JK, Moses, HL, editors. Biomarker tests for molecularly targeted therapies: key to unlocking precision medicine. Washington (DC): National Academies Press (US); 2016.
Prasad V, Fojo T, Brada M (2016) Precision oncology: origins, optimism, and potential. Lancet Oncol 17:e81–e86. doi:10.1016/S1470-2045(15)00620-8
Shames DS, Wistuba II (2014) The evolving genomic classification of lung cancer. J Pathol 232:121–133
Al-Zaid T, Somaiah N, Lazar AJ (2014) Targeted therapies for sarcomas: new roles for the pathologist. Histopathology 64:119–133
Calabrese F, Lunardi F, Popper H (2015) Molecular diagnosis in lung diseases. Front Biosci (Landmark Ed) 20:644–688
De Hertogh G, Geboes KP (2010) Practical and molecular evaluation of colorectal cancer: new roles for the pathologist in the era of targeted therapy. Arch Pathol Lab Med 134:853–863
Dietel M, Sers C (2006) Personalized medicine and development of targeted therapies: the upcoming challenge for diagnostic molecular pathology. A review. Virchows Arch 448:744–755
Flynn C, James J, Maxwell P, et al. (2014) Integrating molecular diagnostics into histopathology training: the Belfast model. J Clin Pathol 67:632–636
Tobin NP, Foukakis T, De Petris L, Bergh J (2015) The importance of molecular markers for diagnosis and selection of targeted treatments in patients with cancer. J Intern Med 278:545–570
Crockford A, Jamal-Hanjani M, Hicks J, Swanton C (2014) Implications of intratumour heterogeneity for treatment stratification. J Pathol 232:264–273
Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C (2015) Translational implications of tumor heterogeneity. Clin Cancer Res 21:1258–1266
Alix-Panabières C, Pantel K (2013) Circulating tumor cells: liquid biopsy of cancer. Clin Chem 59:110–118
Alix-Panabières C, Pantel K (2016) Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 6:479–491
Hofman V, Ilie M, Long E, et al. (2014) Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr Mol Med 14:440–456
Ilie M, Long E, Hofman V, et al. (2014) Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br J Cancer 110:1236–1243
Ilie M, Hofman V, Long E, et al. (2014) Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med 2:107. doi:10.3978/j.issn.2305-5839.2014.08.11
Alix-Panabières C, Pantel K (2014) Technologies for detection of circulating tumor cells: facts and vision. Lab Chip 14:57–62
Haber DA, Velculescu VE (2014) Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 4:650–661
Heitzer E, Ulz P, Geigl JB (2015) Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem 61:112–123
Ignatiadis M, Dawson SJ (2014) Circulating tumor cells and circulating tumor DNA for precision medicine: dream or reality? Ann Oncol 25:2304–2313
Pantel K, Diaz LA Jr, Polyak K (2013) Tracking tumor resistance using ‘liquid biopsies’. Nat Med 19:676–677
Pantel K, Alix-Panabières C (2013) Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res 73:6384–6388
Pantel K, Speicher MR (2015) The biology of circulating tumor cells. Oncogene. doi:10.1038/onc.2015.192
Alix-Panabières C, Pantel K (2014) Challenges in circulating tumour cell research. Nat Rev Cancer 14:623–631
Alix-Panabières C, Bartkowiak K, Pantel K (2016) Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol Oncol
Pantel K, Alix-Panabières C (2016) Liquid biopsy: potential and challenges. Mol Oncol. doi:10.1016/j.molonc.2016.01.009
Pantel K, Alix-Panabières C (2016) Functional studies on viable circulating tumor cells. Clin Chem 62:328–334
Schlange T, Pantel K (2016) Potential of circulating tumor cells as blood-based biomarkers in cancer liquid biopsy. Pharmacogenomics 17:183–186
Bozec A, Ilie M, Dassonville O, et al. (2013) Significance of circulating tumor cell detection using the CellSearch system in patients with locally advanced head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 270:2745–2749
Doyen J, Alix-Panabières C, Hofman P, et al. (2012) Circulating tumor cells in prostate cancer: a potential surrogate marker of survival. Crit Rev Oncol Hematol 81:241–256
Hofman V, Ilie MI, Long E, et al. (2011) Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 129:1651–1660
Ferreira MM, Ramani VC, Jeffrey SS (2016) Circulating tumor cell technologies. Mol Oncol 10:374–394
Hofman VJ, Ilie M, Hofman PM (2015) Detection and characterization of circulating tumor cells in lung cancer: why and how? Cancer Cytopathol. doi:10.1002/cncy.21651
Paterlini-Brechot P, Benali NL (2007) Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 253:180–204
Morrow CJ, Trapani F, Metcalf RL, et al (2016) Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: a clinical case study. Ann Oncol
Pantel K, Denève E, Nocca D, et al. (2012) Circulating epithelial cells in patients with benign colon diseases. Clin Chem 58(5):936–940
Joosse SA, Pantel K (2013) Biologic challenges in the detection of circulating tumor cells. Cancer Res 73:8–11
Brouwer A, De Laere B, Peeters D, et al. (2016) Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget. doi:10.18632/oncotarget.8015
Paoletti C, Hayes DF (2016) Circulating tumor cells. Adv Exp Med Biol 882:235–258
Hofman V, Long E, Ilie M, et al. (2012) Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 23:30–38
Hofman VJ, Ilie MI, Bonnetaud C, et al. (2011) Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol 135:146–156
Hofman V, Bonnetaud C, Ilie MI, et al. (2011) Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 17:827–835
Long E, Ilie M, Bence C, et al. (2016) High expression of TRF2, SOX10, and CD10 in circulating tumor microemboli detected in metastatic melanoma patients. A potential impact for the assessment of disease aggressiveness. Cancer Med. doi:10.1002/cam4.661
Lianidou ES (2016) Gene expression profiling and DNA methylation analyses of CTCs. Mol Oncol 10:431–442
Wicha MS, Hayes DF (2011) Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol 29:1508–1511
Hodgkinson CL, Morrow CJ, Li Y, et al. (2014) Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 20:897–903
Normanno N, De Luca A, Gallo M, Chicchinelli N, Rossi A (2016) The prognostic role of circulating tumor cells in lung cancer. Expert Rev Anticancer Ther 16:859–867
Cai X, Janku F, Zhan Q, Fan JB (2015) Accessing genetic information with liquid biopsies. Trends Genet 31:564–575
Gezer U, Mert U, Ozgür E, Yörüker EE, Holdenrieder S, Dalay N (2012) Correlation of histone methyl marks with circulating nucleosomes in blood plasma of cancer patients. Oncol Lett 3:1095–1098
Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J (2016) Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell 164:57–68
Bordi P, Del Re M, Danesi R, Tiseo M (2015) Circulating DNA in diagnosis and monitoring EGFR gene mutations in advanced non-small cell lung cancer. Transl Lung Cancer Res 4:584–597
Tan CS, Cho BC, Soo RA (2016) Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer. Lung Cancer 93:59–68
Chang GA, Tadepalli JS, Shao Y, et al. (2016) Sensitivity of plasma BRAF (mutant) and NRAS(mutant) cell-free DNA assays to detect metastatic melanoma in patients with low RECIST scores and non-RECIST disease progression. Mol Oncol 10:157–165
De Mattos-Arruda L, Caldas C (2015) Cell-free circulating tumour DNA as a liquid biopsy in breast cancer. Mol Oncol. doi:10.1016/j.molonc.2015.12.001
Diaz LA Jr, Bardelli A (2014) Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 32:579–586
Molina-Vila MA, de-Las-Casas CM, Bertran-Alamillo J, Jordana-Ariza N, González-Cao M, Rosell R (2015) cfDNA analysis from blood in melanoma. Ann Transl Med 3:309. doi:10.3978/j.issn.2305-5839.2015.11.23
Schiavon G, Hrebien S, Garcia-Murillas I, et al. (2015) Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 7:313ra182
Schwaederle M, Husain H, Fanta PT, et al. (2016) Detection rate of actionable mutations in diverse cancers using a biopsy-free (blood) circulating tumor cell DNA assay. Oncotarget. doi:10.18632/oncotarget.7110
Siravegna G, Bardelli A (2015) Blood circulating tumor DNA for non-invasive genotyping of colon cancer patients. Mol Oncol 10:475–480
Schreuer M, Meersseman G, Van Den Herrewegen S, et al. (2016) Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med 14:95. doi:10.1186/s12967-016-0852-
Benesova L, Belsanova B, Suchanek S (2013) Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal Biochem 433:227–234
Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A (2013) Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 10:472–484
Diehl F, Schmidt K, Choti MA, et al. (2008) Circulating mutant DNA to assess tumor dynamics. Nat Med 2008(14):985–890
Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G (2016) Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther 157:120–124
Huang SK, Hoon DS (2015) Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients. Mol Oncol. doi:10.1016/j.molonc.2015.12.008
Lianos GD, Mangano A, Kouraklis G, Roukos DH (2014) Dynamic sequencing of circulating tumor DNA: novel noninvasive cancer biomarker. Biomark Med 8:629–632
Marzese DM, Hirose H, Hoon DS (2013) Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev Mol Diagn 13:827–844
Siravegna G, Bardelli A (2014) Genotyping cell-free tumor DNA in the blood to detect residual disease and drug resistance. Genome Biol 15:449
Tissot C, Toffart AC, Villar S, et al. (2015) Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J 46:1773–1780
Nilsson RJ, Karachaliou N, Berenguer J, et al. (2016) Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget 7(1):1066–1075
Schwarzenbach H, Nishida N, Calin GA, Pantel K (2014) Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol 11:145–156
Pinzani P, Salvianti F, Orlando C, Pazzagli M (2014) Circulating cell-free DNA in cancer. Methods Mol Biol 1160:133–145
Newman AM, Bratman SV, To J, et al. (2014) An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 20:548–554
Sacher AG, Paweletz C, Dahlberg SE, et al. (2016) Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. doi:10.1001/jamaoncol.2016.0173
Sundaresan TK, Sequist LV, Heymach JV, et al. (2016) Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res 22:1103–1110
Thress KS, Brant R, Carr TH, et al. (2015) EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 90:509–515
Watanabe M, Kawaguchi T, Isa S, et al. (2015) Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res 21:3552–3560
Long-Mira E, Washetine K, Hofman P (2016) Sense and nonsense in the process of accreditation of a pathology laboratory. Virchows Arch 468:43–49
Tembuyser L, Dequeker EM (2016) Endorsing good quality assurance practices in molecular pathology: risks and recommendations for diagnostic laboratories and external quality assessment providers. Virchows Arch 468:31–41
Gray ES, Rizos H, Reid AL, et al. (2015) Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 6:42008–41018
Meador CB, Lovly CM (2015) Liquid biopsies reveal the dynamic nature of resistance mechanisms in solid tumors. Nat Med 21:663–665
Hofman V, Ilie M, Long-Mira E, et al. (2013) Usefulness of immunocytochemistry for the detection of the BRAF(V600E) mutation in circulating tumor cells from metastatic melanoma patients. J Invest Dermatol 133:1378–1381. doi:10.1038/jid.2012.485
Ilie M, Long E, Butori C, Hofman V, et al. (2012) ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol 23:2907–2913
Ilie M, Hofman V, Long E, et al. (2016) PD-L1 expression in primary tumor and circulating tumor cells in patients with small cell lung carcinomas. AACR, New Orleans abstract N°
Ilie M, Szafer-Glusman E, Hofman V, et al (2016) PD-L1 expression in primary tumor and circulating tumor cells in patients with advanced non-small cell lung cancer. Virchows Archiv ECP abstract N°. XXI International Congress of the International Academy of Pathology and 28 th Congress of the European Society of Pathology, Cologne, Germany. Thoracic Pathology OFP-13, 003. Virchow Archivs 2016, in press
Pailler E, Auger N, Lindsay CR, et al. (2015) High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann Oncol 26:1408–1415
Ilie M, Hofman V, Long-Mira E, et al. (2014) “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One 9:e111597. doi:10.1371/journal.pone.0111597
Hofman P (2015) Search for circulating tumor cells: seriously, a real cancer screening tool? J Mal Vasc 40:335–337
Jamal-Hanjani M, Wilson GA, Horswell S, et al. (2016) Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol 27:862–867
Montani F, Marzi MJ, Dezi F, et al. (2015) miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst 107:djv063. doi:10.1093/jnci/djv063
Lebofsky R, Decraene C, Bernard V, et al. (2015) Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol Oncol 9:783–790
Tsui DW, Berger MF (2016) Profiling non-small cell lung cancer: from tumor to blood. Clin Cancer Res 22:790–792
Ziegler A, Zangemeister-Wittke U, Stahel RA (2002) Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev 28:255–271
Salgia R (2015) Diagnostic challenges in non-small-cell lung cancer: an integrated medicine approach. Future Oncol 11:489–500
Schumacher TN, Scheper W (2016) A liquid biopsy for cancer immunotherapy. Nat Med 22:340–341
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare to respect all ethical standards.
Funding
None
Conflict of interest
PH declares receiving honoraria from pharmaceutical (AstraZeneca, Roche, Novartis, Bristol Myers Squibb, MSD) and biotechnology (Qiagen, Janssen, Biocartis) companies for advisory board meetings. HP declares receiving honoraria from pharmaceutical companies for advisory board meeting and also unrestricted grants for research (Bristol Myers Squibb, Eli Lilly, Roche, Boehringer-Ingelheim, Astra Zeneca, Novartis).
Rights and permissions
About this article
Cite this article
Hofman, P., Popper, H.H. Pathologists and liquid biopsies: to be or not to be?. Virchows Arch 469, 601–609 (2016). https://doi.org/10.1007/s00428-016-2004-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-2004-z