Abstract
Background
Uveal melanoma is the most common primary ocular malignancy in adults in the USA and Europe. The optimal treatment of large uveal melanoma is still under debate. Radiation therapy has its limitation due its eye-threatening secondary complications and is therefore often combined with surgical excision of the tumor.
Methods
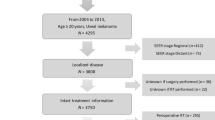
In a retrospective interventional review, we evaluated in total 242 patients with uveal melanoma that underwent transscleral tumor resection with a predefined protocol, either with adjuvant ruthenium brachytherapy (Ru-106 group, n 136,), or with neoadjuvant proton beam therapy (PBT group, n 106). Kaplan-Meier estimates with log-rank test were used to show survival curves and a multivariable Cox regression model was used to calculate adjusted rate ratios.
Results
Local tumor recurrence rates after 3 and 5 years were 4% (95% CI 1.2–17.8%) and 9.1% (95% CI 2.9–27.3%), respectively, in the PBT group and 24.6% (95% CI 15.8–37.1%) and 27.5 (95% CI 17.8–41.1%), respectively, in the Ru-106 group. This leads to an overall recurrence rate almost 4 times higher in the Ru-106 group compared to the PBT group. After adjusting for the a priori confounders and the tumor distance to optic disc and ciliary body infiltration, the adjusted risk of tumor recurrence was 8 times (RR 7.69 (2.22–26.06), p < 0.001) higher in the Ru-106 group as compared to the PBT group. Three- and 5-year metastatic rates were 23.2% (95% CI 5.6–37.1%) and 31.8% (95% CI 20.7–46.8%), respectively, in the PBT group and 13.2% (95% CI 6.8–24.9%) and 30.3% (95% CI 18.3–47.5%), respectively, in the Ru-106 group. There was no statistically significant difference in the overall metastasis rate between the two groups even after adjusting for possible confounders.
Conclusion
Transscleral resection of large uveal melanomas combined with neoadjuvant proton beam therapy leads to a lower local tumor recurrence rate compared to transscleral tumor resection with adjuvant ruthenium brachytherapy. There was no statistically significant difference in the occurrence of rubeosis iridis, neovascular glaucoma, and in the need for enucleation later on.



Similar content being viewed by others
References
Collaborative Ocular Melanoma Study G (2006) The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report no. 28. Arch Ophthalmol 124:1684–1693. https://doi.org/10.1001/archopht.124.12.1684
Lommatzsch PK (1986) Results after beta-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years experience. Br J Ophthalmol 70:844–851
Puusaari I, Heikkonen J, Summanen P, Tarkkanen A, Kivelä T (2003) Iodine brachytherapy as an alternative to enucleation for large uveal melanomas. Ophthalmology 110:2223–2234. https://doi.org/10.1016/S0161-6420(03)00661-4
Conway R, Poothullil AM, Daftari IK, Weinberg V, Chung JE, O’Brien JM (2006) EStimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapy. Arch Ophthalmol 124:838–843. https://doi.org/10.1001/archopht.124.6.838
Lumbroso L, Desjardins L, Levy C, Plancher C, Frau E, D’Hermies F, Schlienger P, Mammar H, Delacroix S, Nauraye C, Ferrand R, Desblancs C, Mazal A, Asselain B (2001) Intraocular inflammation after proton beam irradiation for uveal melanoma. Br J Ophthalmol 85:1305–1308. https://doi.org/10.1136/bjo.85.11.1305
Saornil MA, Egan KM, Gragoudas ES, Seddon JM, Walsh SM, Albert DM (1992) HIstopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol 110:1112–1118. https://doi.org/10.1001/archopht.1992.01080200092031
Muller HK, Lund OE, Sollner F, Seidel G (1966) The operative treatment of tumors of the chamber angle and ciliary body. Doc Ophthalmol 20:500–518
Stallard HB (1966) Partial choroidectomy. Br J Ophthalmol 50:660–662
Bechrakis NE, Petousis V, Willerding G, Krause L, Wachtlin J, Stroux A, Foerster MH (2010) Ten-year results of transscleral resection of large uveal melanomas: local tumour control and metastatic rate. Br J Ophthalmol 94:460–466. https://doi.org/10.1136/bjo.2009.162487
Damato BE, Paul J, Foulds WS (1996) Risk factors for metastatic uveal melanoma after trans-scleral local resection. Br J Ophthalmol 80:109–116
Kivelä T, Puusaari I, Damato B (2003) Transscleral resection versus iodine brachytherapy for choroidal malignant melanomas 6 millimeters or more in thickness: a matched case–control study. Ophthalmology 110:2235–2244. https://doi.org/10.1016/j.ophtha.2003.02.001
Willerding GD, Cordini D, Moser L, Krause L, Foerster MH, Bechrakis NE (2015) Neoadjuvant proton beam irradiation followed by transscleral resection of uveal melanoma in 106 cases. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2015-307095
Foulds WS (1973) The local excision of choroidal melanomata. Trans Ophthalmol Soc U K 93:343–346
Foulds WS (1995) Local resection and other conservative therapies for intraocular melanoma. Curr Opin Ophthalmol 6:62–69
Damato B, Groenewald CP, McGalliard JN, Wong D (2002) Rhegmatogenous retinal detachment after transscleral local resection of choroidal melanoma. Ophthalmology 109:2137–2143
Bechrakis NE, Bornfeld N, Zöller I, Foerster MH (2002) Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 109:1855–1861. https://doi.org/10.1016/S0161-6420(02)01273-3
Barbara D, Rolf B (2002) Precise modelling of the eye for proton therapy of intra-ocular tumours. Phys Med Biol 47:593
Marnitz S, Cordini D, Bendl R, Lemke A-J, Heufelder J, Simiantonakis I, Kluge H, Bechrakis NE, Foerster MH, Hinkelbein W (2006) Proton therapy of uveal melanomas. Strahlenther Onkol 182:395–399. https://doi.org/10.1007/s00066-006-1512-1
Goitein M, Miller T (1983) Planning proton therapy of the eye. Med Phys 10:275–283. https://doi.org/10.1118/1.595258
Heufelder J, Cordini D, Fuchs H, Heese J, Homeyer H, Kluge H, Morgenstern H, Hocht S, Nausner M, Bechrakis NE, Hinkelbein W, Foerster MH (2004) Five years of proton therapy of eye neoplasms at the Hahn-Meitner Institute, Berlin. Z Med Phys 14:64–71
Damato BE (2012) Local resection of uveal melanoma. Dev Ophthalmol 49:66–80. https://doi.org/10.1159/000328261
Gallie BL, Simpson ER, Saakyan S, Amiryan A, Valskiy V, Finger PT, Chin KJ, Semenova E, Seregard S, Fili M, Wilson M, Haik B, Caminal JM, Català J, Gutierrez C, Pelayes DE, Folgar AM, Jager MJ, Dogrusöz M, Luyten GPM, Singh A, Schachat AP, Suzuki S, Aihara Y (2016) Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology 123:86–91. https://doi.org/10.1016/j.ophtha.2015.09.014
Konstantinidis L, Groenewald C, Coupland SE, Damato B (2014) Trans-scleral local resection of toxic choroidal melanoma after proton beam radiotherapy. Br J Ophthalmol 98:775–779. https://doi.org/10.1136/bjophthalmol-2013-304501
Daftari IK, Char DH, Verhey LJ, Castro JR, Petti PL, Meecham WJ, Kroll S, Blakely EA (1997) Anterior segment sparing to reduce charged particle radiotherapy complications in uveal melanoma. Int J Radiat Oncol Biol Phys 39:997–1010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights and informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Böker, A., Pilger, D., Cordini, D. et al. Neoadjuvant proton beam irradiation vs. adjuvant ruthenium brachytherapy in transscleral resection of uveal melanoma. Graefes Arch Clin Exp Ophthalmol 256, 1767–1775 (2018). https://doi.org/10.1007/s00417-018-4032-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4032-7