Abstract
Purpose
To evaluate the agreement and predictability of ocriplasmin treatment effects among retinal experts (raters) by assessment of retinal imaging data of eyes treated for vitreomacular traction in nine different centers in Germany and Austria.
Methods
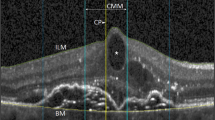
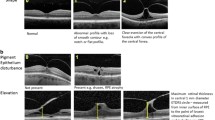
Retrospective cohort study. Combined confocal near-infrared scanning laser ophthalmoscopy and spectral-domain optical coherence tomography images (Spectralis® device, Heidelberg Engineering GmbH, Germany) from 136 eyes of 135 subjects were reviewed by 14 raters using an internet-based grading database and a standardized questionnaire. In addition to the images taken within 2 days prior to treatment, age, gender, and lens status were disclosed to the raters. Treatment success was defined as a complete cleavage of the posterior vitreous cortex at day 28±5. Main outcome was the agreement and predictability among raters for assessment of treatment success.
Results
Raters generally accepted starting ocriplasmin treatment (chance for treatment success ≥ 1%) in 22.4 to 69.1% (median 53.2%) of eyes (moderate intra- and interrater agreements with kappa-values of 0.6 and 0.48). The likelihood for a high potential treatment success (equal or higher than 25%) was judged by the raters in 43.4% to 86.0% (median 62.6%) of eyes (moderate intra- and fair interrater agreements with kappa-values of 0.56 and 0.22). Allocating eyes for high potential treatment success overall increased the odds by 3.07, with odds ratios of single raters up to 4.06 to 6.16.
Conclusions
These results underscore the importance of training health care providers in the evaluation of retinal imaging data and also to define characteristic morphological features better in the presence of vitreoretinal interface diseases. The better results of single raters in the predictability of treatment success by the allocation of eyes in the high-potential group indicates the high relevance of the meticulous analysis of retinal images.





Similar content being viewed by others
References
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm324369.htm (Accessed on 06 Apr 2016)
http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002381/WC500142159.pdf (Accessed on 06 Apr 2016)
García-Layana A, García-Arumí J, Ruiz-Moreno JM, Arias-Barquet L, Cabrera-López F, Figueroa MS (2015) A review of current management of vitreomacular traction and macular hole. J Ophthalmol 2015:809640. doi:10.1155/2015/809640
Khan MA, Haller JA (2015) Clinical management of vitreomacular traction. Curr Opin Ophthalmol 26:143–148. doi:10.1097/ICU.0000000000000149
Stellungnahme der Deutschen Ophthalmologischen Gesellschaft, der Retinologischen Gesellschaft und des Berufsverbandes der Augenärzte Deutschlands: Die Anti-VEGF-Therapie bei der neovaskulären altersabhängigen Makuladegeneration: Therapeutische Strategien. www.dog.org (Accessed on 06 Apr 2016)
Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, Haller JA, Group M-TS (2012) Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med 367:606–615. doi:10.1056/NEJMoa1110823
Haller JA, Stalmans P, Benz MS, Gandorfer A, Pakola SJ, Girach A, Kampik A, Jaffe GJ, Toth CA, Group M-TS (2015) Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 122:117–122. doi:10.1016/j.ophtha.2014.07.045
Chatziralli I, Theodossiadis G, Parikakis E, Datseris I, Theodossiadis P (2015) Real-life experience after intravitreal ocriplasmin for vitreomacular traction and macular hole: a spectral-domain optical coherence tomography prospective study. Graefes Arch Clin Exp Ophthalmol. doi:10.1007/s00417-015-3031-1
Yamamoto S, Yamamoto T, Ogata K, Hoshino A, Sato E, Mizunoya S (2004) Morphological and functional changes of the macula after vitrectomy and creation of posterior vitreous detachment in eyes with diabetic macular edema. Doc Ophthalmol 109:249–253
Sharma P, Juhn A, Houston SK, Fineman M, Chiang A, Ho A, Regillo C (2015) Efficacy of Intravitreal Ocriplasmin on Vitreomacular Traction and Full-Thickness Macular Holes. Am J Ophthalmol. doi:10.1016/j.ajo.2015.01.034
Maier M, Abraham S, Frank C, Feucht N, Lohmann CP (2015) Ocriplasmin as a treatment option for symptomatic vitreomacular traction with and without macular hole: First clinical experiences. Ophthalmologe. doi:10.1007/s00347-015-0073-z
Lommatzsch AP, Gutfleisch M, Dietzel M, Heimes B, Spital G, Böhme M, Bornfeld N, Pauleikhoff D (2014) Initial clinical experience in the treatment of vitreomacular traction and macular holes with ocriplasmin. Klin Monbl Augenheilkd 231:909–914. doi:10.1055/s-0034-1368372
Nudleman E, Franklin MS, Wolfe JD, Williams GA, Ruby AJ (2015) RESOLUTION OF SUBRETINAL FLUID AND OUTER RETINAL CHANGES IN PATIENTS TREATED WITH OCRIPLASMIN. Retina. doi:10.1097/iae.0000000000000755
Mennel S (2014) Enzymatic vitreolysis – from clinical trials to clinical practice. Spektrum Augenheilkd. 28:59–60
Singh RP, Li A, Bedi R, Srivastava S, Sears JE, Ehlers JP, Schachat AP, Kaiser PK (2014) Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol 98:356–360. doi:10.1136/bjophthalmol-2013-304219
Kaiser PK, Kampik A, Kuppermann BD, Girach A, Rizzo S, Sergott RC (2015) Safety profile of ocriplasmin for the pharmacologic treatment of symptomatic vitreomacular adhesion/traction. Retina 35:1111–1127. doi:10.1097/iae.0000000000000448
Geck U, Pustolla N, Baraki H, Atili A, Feltgen N, Hoerauf H (2013) Posterior vitreous detachment following intravitreal drug injection. Graefes Arch Clin Exp Ophthalmol 251:1691–1695. doi:10.1007/s00417-013-2266-y
Yamamoto T, Akabane N, Takeuchi S (2001) Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol 132:369–377
Yamamoto T, Kamei M, Kunavisarut P, Suzuki M, Tano Y (2008) Increased retinal toxicity of intravitreal tissue plasminogen activator in a central retinal vein occlusion model. Graefes Arch Clin Exp Ophthalmol 246:509–514. doi:10.1007/s00417-007-0670-x
Paul C, Heun C, Müller HH, Fauser S, Kaymak H, Kazerounian S, Sekundo W, Mennel S, Meyer CH, Schmitz-Valckenberg S, Koss MJ, Feltgen N, Bertelmann T (2016) IMPACT OF VITREORETINAL INTERFACE ARCHITECTURE ON SUCCESSFUL VITREOMACULAR TRACTION RESOLUTION IN EYES SCHEDULED FOR INTRAVITREAL OCRIPLASMIN THERAPY. Retina. doi:10.1097/IAE.0000000000001371
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P (2013) The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 120:2611–2619. doi:10.1016/j.ophtha.2013.07.042
Mastropasqua R, Di Antonio L, Ciciarelli V, Aharrh-Gnama A, Rispoli M, Carpineto P (2016) Comparison of Guided and Unguided Ocriplasmin Injection for the Treatment of Vitreomacular Traction: A Preliminary Study. J Ophthalmol 2016:6521304. doi:10.1155/2016/6521304
EXPORT study group: Thomas Bertelmann (Department of Ophthalmology, University Medical Center Goettingen, Germany), Hans Hoerauf (Department of Ophthalmology, University Medical Center Goettingen, Germany), Nicolas Feltgen (Department of Ophthalmology, University Medical Center Goettingen, Germany), Joachim Wachtlin (Department of Ophthalmology, Sankt Gertrauden-Krankenhaus, Berlin, Germany), Hakan Kaymak (Innovative Internationale Augenchirurgie (IIO), Duesseldorf, Germany), Stefan Mennel (Department of Ophthalmology, Feldkirch State Hospital, Feldkirch, Austria), Michael J. Koss (Augenzentrum Nymphenburger Höfe/Augenklinik Herzog Carl Theodor, Munich, Germany and Department of Ophthalmology, Heidelberg University, Germany), Sascha Fauser (Department of Ophthalmology, University of Cologne, Cologne, Germany), Mathias M. Maier (Department of Ophthalmology, Klinikum rechts der Isar, Technische Universität München, Germany), Ricarda G. Schumann (Department of Ophthalmology, Ludwig-Maximilian-University, Munich, Germany), Simone Müller (GRADE Reading Center and Department of Ophthalmology, University of Bonn, Germany), Petrus Chang (GRADE Reading Center and Department of Ophthalmology, University of Bonn, Germany), Sara Kazerounian (Department of Ophthalmology, Knappschaftskrankenhaus Sulzbach, Germany), Albrecht Lommatzsch (Department of Ophthalmology, St. Franziskus-Hospital, Münster, Germany), Hanna Daniel (Department of Biometry and Medical Epidemiology, Philipps-University, Marburg, Germany), Steffen Schmitz-Valckenberg (GRADE Reading Center and Department of Ophthalmology, University of Bonn, Germany)
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Thomas Bertelmann has received funding for research and clinical trials from Alcon (USA), Alimera Sciences (USA), Allergan (Ireland), Bayer HealthCare (Germany), and Novartis (Switzerland), as well as consulting fees, honoraria, and travel reimbursement from Alcon (USA), Alimera Sciences (USA), Allergan (Ireland), Bayer HealthCare (Germany), and Novartis (Switzerland). He is a scientific staff member of Georg-August-University Goettingen, Germany and a medical advisor for Novartis Pharma GmbH (Nuremberg, Germany).
Joachim Wachtlin has received consulting fees, honoraria, and travel reimbursement from Alcon (USA), Allergan (Ireland), Bayer HealthCare (Germany), and Novartis (Switzerland).
Stefan Mennel received honoraria to the institution for a board membership in Feb 2015 from Bayer HealthCare (Germany) and travel reimbursement from Abbott Medical Optics (USA).
Michael J. Koss declares no conflict of interest.
Mathias M. Maier has participated in clinical trials from Alcon (USA), Bayer (Germany) and Novartis (Switzerland), as well as has received speaker honoraria from Alcon (USA), Allergan (Ireland), Bayer (Germany), Heidelberg Engineering (Germany) and Novartis (Switzerland)
Ricarda G. Schumann has received speaker honoraria and travel expense reimbursement from Alcon (Heidelberg, Germany), Novartis (Nuremberg, Germany), and Allergan (Dublin, Ireland).
Sara Kazerounian declares no conflict of interest.
Hanna Daniel declares no conflict of interest.
Steffen Schmitz-Valckenberg has received funding for research and clinical trials from Alcon (USA), Allergan (Ireland), Bayer (Germany), Bioeq (Germany), Carl Zeiss Meditech (Germany), Genentech (USA), Heidelberg Engineering (Germany), Novartis (Switzerland), Optos (Germany), and Roche (Switzerland), as well as consulting fees and honoraria from Alcon (USA), Bayer (Germany), Heidelberg Engineering (Germany), and Novartis (Germany).
Funding
No funding was received for this research.
Ethical approval
For this type of study formal consent is not required.
Electronic supplementary material
ESM 1.
Export Study Grading Manual (PDF 1805 kb)
Rights and permissions
About this article
Cite this article
Bertelmann, T., Wachtlin, J., Mennel, S. et al. The predictability of ocriplasmin treatment effects: is there consensus among retinal experts? Results from the EXPORT study. Graefes Arch Clin Exp Ophthalmol 255, 1359–1367 (2017). https://doi.org/10.1007/s00417-017-3657-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3657-2