Abstract
Background
Stimulus parameters, in particular pulse shape, are an important consideration in the application of electrical stimulation when experimentally testing a visual prosthesis. We changed the biphasic pulse shape of several asymmetric charge-balanced pulses to investigate their effect on optic nerve (ON) stimulation and the recorded cortical response.
Methods
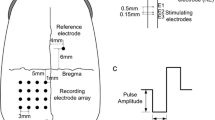
Monopolar platinum–iridium electrodes were implanted into the rabbit’s ON behind the eyeball. Electrical evoked potentials (EEPs) were recorded with silver ball electrodes placed on the cortex, and the results quantified.
Results
Our results indicate that changing the shape of cathodic-first charge-balanced biphasic pulse (CA) while maintaining charge balance could reduce the current thresholds for stimulation. When stimulated at the same charge density, the stimulus having high-amplitude short-duration (HASD) cathodic phase produced a higher amplitude response, with a larger spatial spread but with a lower current threshold compared with other stimuli. Adding an inter-phase gap between the two phases of the stimulus increased the EEP amplitude, but was saturated at a gap of ∼0.2 ms; this was most obvious with CA stimulation, which was able to elicit a larger cortical response than that elicited by asymmetrical charge-balanced stimulus pulses with HASD cathodic phase, in contrast to CA without a gap. As the stimulating frequency increased, the amplitudes of the EEP components elicited by CA monotonically decreased. The fastest component (P0) was present with stimulating frequencies as high as 80 Hz, while the slower P1 and P2 disappeared with stimulating frequencies higher than 40 and 20 Hz, respectively.
Conclusion
A CA stimulus waveform with an inter-phase gap of 0.2 ms was more efficacious for ON stimulation than other stimulus combinations, and therefore should result in less tissue damage, minimal electrode etching, and lower power consumption if used in a visual prosthesis.







Similar content being viewed by others
Reference
Schmidt EM, Bak MJ, Hambrecht FT, Kufta CV, O'Rourke DK, Vallabhanath P (1996) Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain 119:507–522
Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G (2003) Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res 43:2573–2581
Rizzo JF 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D (2003) Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci 44:5355–5361
Kanda H, Morimoto T, Fujikado T, Tano Y, Fukuda Y, Sawai H (2004) Electrophysiological studies of the feasibility of suprachoroidal–transretinal stimulation for artificial vision in normal and RCS rats. Invest Ophthalmol Vis Sci 45:560–566
Kim ET, Kim C, Lee SW, Seo JM, Chung H, Kim SJ (2009) Feasibility of microelectrode array (MEA) based on silicone–polyimide hybrid for retina prosthesis. Invest Ophthalmol Vis Sci 50:4337–4341
Yamauchi Y, Franco LM, Jackson DJ, Naber JF, Ziv RO, Rizzo JF, Kaplan HJ, Enzmann V (2005) Comparison of electrically evoked cortical potential thresholds generated with subretinal or suprachoroidal placement of a microelectrode array in the rabbit. J Neural Eng 2:S48–S56
Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S (2011) Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci 278:1489–1497
da Cruz L, Coley B, Christopher P, Merlini F, Wuyyuru V, Sahel JA, Stanga P, Filley E, Dagnelie G (2010) Patients blinded by outer retinal dystrophies are able to identify letters using the ArgusTM II retinal prosthesis system. Invest Ophthalmol Visual Sci 51: ARVO E-Abstract #2023
Brelén ME, De Potter P, Gersdorff M, Gersdorff M, Cosnard G, Veraart C, Delbeke J (2006) Intraorbital implantation of a stimulating electrode for an optic nerve visual prosthesis. J Neurosurg 104:593–597
Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini S, Wanet-Defalque MC (1998) Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res 813:181–186
Duret F, Brelén ME, Lambert V, Gérard B, Delbeke J, Veraart C (2006) Object localization, discrimination, and grasping with the optic nerve visual prosthesis. Restor Neurol Neurosci 24:31–40
Veraart C, Wanet-Defalque MC, Gérard B, Vanlierde A, Delbeke J (2003) Pattern recognition with the optic nerve visual prosthesis. Artif Organs 27:996–1004
Li L, Cao P, Sun M, Chai X, Wu K, Xu X, Li X, Ren Q (2009) Intraorbital optic nerve stimulation with penetrating electrodes: in vivo electrophysiology study in rabbits. Graefes Arch Clin Exp Ophthalmol 247:349–361
Sun J, Lu Y, Cao P, Li X, Cai C, Chai X, Ren Q, Li L (2010) Spatiotemporal properties of multipeaked electrically evoked potentials elicited by penetrative optic nerve stimulation in rabbits. Invest Ophthalmol Vis Sci 52:146–154
Rosahl SK, Mark G, Herzog M, Pantazis C, Gharabaghi F, Matthies C, Brinker T, Samii M (2001) Far-field responses to stimulation of the cochlear nucleus by microsurgically placed penetrating and surface electrodes in the cat. J Neurosurg 95:845–852
Brummer SB, Turner MJ (1977) Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans Biomed Eng 24:59–63
Rowland V, MacIntyre WJ, Bidder TG (1960) The production of brain lesions with electric currents. II. Bidirectional currents. J Neurosurg 17:55–69
Loucks RB, Weinberg H, Smith M (1959) The erosion of electrodes by small currents. Electroencephalogr Clin Neurophysiol 11:823–826
Shepherd RK (1999) Chronic electrical stimulation of the auditory nerve using non-charge-balanced stimuli. Acta Otolaryngol 119:674–684
Lilly JC, Hughes JR, Alvord EC Jr, Galkin TW (1955) Brief, noninjurious electric waveform for stimulation of the brain. Science 121:468–469
Lilly JC, Cherry RB (1955) Surface movements of figures in spontaneous activity of anesthetized cerebral cortex: leading and trailing edges. J Neurophysiol 18:18–32
Lilly JC (1961) Injury and excitation by electric currents. In: Electrical stimulation of the brain. University of Texas Press, Austin, pp 60–66
Van Wieringen A, Macherey O, Carlyon RP, Deeks JM, Wouters J (2008) Alternative pulse shapes in electrical hearing. Hear Res 242:154–163
Macherey O, Van Wieringen A, Carlyon RP, Deeks JM, Wouters J (2006) Asymmetric pulses in cochlear implants: Effects of pulse shape, polarity, and rate. J Assoc Res Otolaryngol 7:253–266
Choudhury BP (1987) Visual cortex in the albino rabbit. Exp Brain Res 66:565–571
Burke W, Cottee LJ, Garvey J, Kumarasinghe R, Kyriacou C (1986) Selective degeneration of optic nerve fibres in the cat produced by a pressure block. J Physiol 376:461–476
Burke W, Burne JA, Martin PR (1985) Selective block of Y optic nerve fibres in the cat and the occurrence of inhibition in the lateral geniculate nucleus. J Physiol 364:81–92
Rizzo JF 3rd, Goldbaum S, Shahin M, Denison TJ, Wyatt J (2004) In vivo electrical stimulation of rabbit retina with a microfabricated array: strategies to maximize responses for prospective assessment of stimulus efficacy and biocompatibility. Restor Neurol Neurosci 22:429–443
Miller CA, Abbas PJ, Robinson BK, Rubinstein JT, Matsuoka AJ (1999) Electrically evoked single-fiber action potentials from cat: responses to monopolar, monophasic stimulation. Hear Res 130:197–218
Ranck JB (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98:417–440
Miller CA, Robinson BK, Rubinstein JT, Abbas PJ, Runge-Samuelson CL (2001) Auditory nerve responses to monophasic and biphasic electric stimuli. Hear Res 151:79–94
van den Honert C, Mortimer JT (1979) The response of the myelinated nerve fiber to short duration biphasic stimulating currents. Ann Biomed Eng 7:117–125
Grill WM, Mortimer JT (1995) Stimulus waveforms for selective neural stimulation. IEEE Eng Med Biol Mag 14:375–385
Van Wieringen A, Carlyon RP, Laneau J, Wouters J (2005) Effects of waveform shape on human sensitivity to electrical stimulation of the inner ear. Hear Res 200:73–86
Grill WM, Mortimer JT (1996) The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans Biomed Eng 43:161–166
Shepherd RK, Javel E (1999) Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res 130:171–188
Prado-guitierrez P, Fewster LM, Heasman JM, Mckay CM, Shepherd RK (2006) Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res 215:47–55
Delbeke J, Oozeer M, Veraart C (2003) Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vision Res 43:1091–1102
Acknowledgements
This research is supported by The National Basic Research Program of China(973 Program, 2011CB707502/3); The National Natural Science Foundation of China (60971102, 31070981, 61171174, 91120304). The authors thank Dr. T. FitzGibbon for comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, J., Chen, Y., Chai, X. et al. Penetrating electrode stimulation of the rabbit optic nerve: parameters and effects on evoked cortical potentials. Graefes Arch Clin Exp Ophthalmol 251, 2545–2554 (2013). https://doi.org/10.1007/s00417-013-2449-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2449-6