Abstract
Background
Intracameral moxifloxacin is currently administered by injecting small doses (0.05–0.2 mL) of either undiluted or diluted solutions. It is difficult to ensure delivery of small amounts of antibiotic into the area behind the intraocular lens (IOL). Moreover, the anterior chamber pressure decreases as the tip of irrigation is removed, often leading to contaminated fluid flowing into the chamber. Conventional intracameral injection administers the diluted antibiotic without irrigating the recontaminated anterior chamber. Therefore, we developed a method of intracameral moxifloxacin delivery which flushes both the anterior chamber and the area behind the IOL immediately after surgery.
Methods
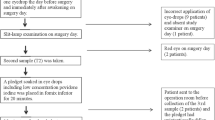
Surgical technique (bag and chamber flushing = BC flushing): After removing the viscosurgical device, 1.5–1.8 mL diluted moxifloxacin was injected. Both the anterior chamber and the area behind the IOL were irrigated by lifting the IOL edge so that a stream of solution could circulate behind the IOL. Experiment 1 (pig): The anterior chamber was filled with condensed milk, and irrigated with 150-fold diluted moxifloxacin (33.3 μg/mL) in six eyes (BC flushing) to observe the irrigating effect. The anterior aqueous humor was sampled. Experiment 2 (human): A conventional intracameral injection (500 μg/mL) or BC flushing (33.3 μg/mL) was followed by sampling 0.1 mL of the anterior aqueous humor in six eyes each. High-performance liquid chromatography was performed to determine antibiotic levels.
Results
Experiment 1: The antibiotic concentration in the anterior chamber was 33.0 μg/mL (99.0 % was displaced). The area behind the IOL was not effectively irrigated without inserting the cannula tip. Experiment 2: The final antibiotic concentration was 152.3 μg/mL using the conventional method and 29.4 μg/mL using the BC flushing (88.3 % was displaced).
Conclusion
BC flushing technique enables surgeons to completely displace the anterior chamber including the posterior IOL surface, resulting in effective irrigation and a stable antibiotic concentration in virtually all cases.





Similar content being viewed by others
References
John T, Sims M, Hoffman C (2000) Intraocular bacterial contamination during sutureless, small incision, single-port phacoemulsification. J Cataract Surg 26:1786–1792
Tervo T, Ljungberg P, Kautiainen T, Pusk P, Leht I, Raivio I, Järvinen E, Kuusela P, Tarkkanen A (1999) Prospective evaluation of external ocular microbial growth and aqueous humor contamination during cataract surgery. J Cataract Refract Surg 25:65–71
Wada T, Kozai S, Tajika T, Sakai H, Suzuki T, Ohashi Y (2008) Prophylatic efficacy of ophthalmic quinolones in experimental endophthalmitis in rabbits. J Ocul Pharmacol Ther 24:278–289
Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson R (2005) Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg 31:735–741
Endophthalmitis Surgery Group, European Society of Cataract & Refractive Surgeons (2007) Prophylaxis of postoperative endophthalmitis following cataract surgery: result of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 33:978–988
Espiritu CR, Ramon CG, Caparas VL, Bolinao JG (2007) Safety of prophylactic intracameral moxifloxacin 0.5 % ophthalmic solution in cataract patients. J Cataract Refract Surg 33:63–68
Kim SY, Park YH, Lee YC (2008) Comparison of the effect of intracameral moxifloxacin and cefazolin on rabbit corneal endothelial cells. Clin Experiment Ophthalmol 36:367–370
Lane SS, Osher RH, Masket S, Belani S (2008) Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg 34:1451–1459
O’Brien TP, Arshinoff SA, Mah FS (2007) Perspectives on antibiotics for postoperative endopthalmitis prophylaxis: potential role of moxifloxacin. J Cataract Refract Surg 33:1790–1800
Suzuki T, Wada T, Kozai S, Ike Y, Gilmore MS, Ohashi Y (2008) Contribution of secreted proteases to the pathogenesis of postoperative Enterococcus faecalis endophthalmitis. J Cataract Refract Surg 34:1776–1784
Kernt M, Neubauer AS, Liegl RG, Lackerbauer CA, Eibl KH, Alge CS, Ulbig MW, Kampik A (2009) Intracameral moxifloxacin: In vitro safety on human ocular cells. Cornea 28:553–561
Mather R, Karenchak LM, Romanowski EG, Kowalski EG (2002) Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol 133:463–466
Blondeau JM (1999) A review of comparative in-vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolones. J Antimicrob Chemother 43(suppl B):1–11
Kowalski RP, Dhaliwal DK, Karenchak LM, Romanpwski EG, Mah FS, Ritterband DC, Gordon YJ (2003) Gatifloxacin and moxifloxacin; an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am J Ophthalmol 136:500–505
Kodjikian L, Renaud FN, Roques C, Garweg JG, Pellon G, Freney J, Burillon C (2005) In vitro influence of vancomycin on adhesion of a Staphylococcus epidermidis strain encoding intracellular adhesion locus ica to intraocular lenses. J Cataract Refract Surg 31:1050–1058
Acknowledgment
The authors would like to thank Mr. Mitsuharu Ikeda and Dr. Shinichi Sasaki for assistance with the manuscript.
Author disclosure statement
No competing financial interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuura, K., Suto, C., Akura, J. et al. Bag and chamber flushing: a new method of using intracameral moxifloxacin to irrigate the anterior chamber and the area behind the intraocular lens. Graefes Arch Clin Exp Ophthalmol 251, 81–87 (2013). https://doi.org/10.1007/s00417-012-2098-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-2098-1