Abstract
Background
Complicated approval procedures and limited short-term surgical capacities can result in time delays between the definition of a medical indication for ranibizumab treatment in active neovascular age-related macular degeneration (AMD) and the starting of treatment. This study aimed to evaluate changes in visual acuity and central retinal thickness over time, and their consequences for the patients concerned.
Methods
Sixty-nine patients indicated for first-time ranibizumab treatment and 21 patients with necessary re-treatment were included in the study. Visual acuity and spectral domain optical coherence tomography (SD-OCT) central retinal thickness at the time of the indication examination were compared to values at the first-time treatment and during recurrent ranibizumab treatment.
Results
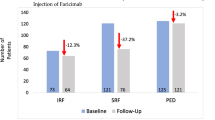
For first-time treatment, the delay between indication and treatment was significantly higher for patients with vision loss compared to those without vision loss (31.6 ± 20.5 vs. 24.0 ± 8.3 days, p = 0.012). The increase in OCT central retinal thickness was 50.4 ± 92.8 μm for patients with vision loss compared to 5.1 ± 63.4 μm for those without vision loss, p = 0.029. A 1.1 logMAR line difference in vision loss was significant at p = 0.01 for patients with a delay in treatment of less than or equal to 28 days (48/69 patients, 69.7%) compared to those with a delay of more than 28 days (21/69 patients, 30.3%).
Conclusions
Even though average visual decay was slow at about one logMAR line over 110 days, individual patients (8.7%) experienced rapid loss of one or more lines within 21 days. Administrative procedures should therefore be expedited so that delays do not exceed 2 weeks for the sake of vision preservation in individual patients.




Similar content being viewed by others
References
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. doi:10.1056/NEJMoa062655
Brown MM, Brown GC, Brown HC, Peet J (2008) A value-based medicine analysis of ranibizumab for the treatment of subfoveal neovascular macular degeneration. Ophthalmology 115:1039–1045
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. doi:10.1056/NEJMoa054481
EMEA (2007) Lucentis Combined Product Information. In: EMEA (ed) emea-combined-h715enpdf
EMEA (2007) EMEA Scientific discussion LUCENTIS. In: EMEA (ed) H-715-en6, pp 54
EMEA (2008) European Public Assessment Report (EPAR) LUCENTIS. In: EMEA (ed) EMEA/H/C/715, pp 54
Arias L, Armada F, Donate J, Garcia-Arumi J, Giralt J, Pazos B, Pinero A, Martinez F, Mondejar JJ, Ortega I, Zlateva G, Buggage R (2009) Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye (Lond) 23:326–333. doi:10.1038/sj.eye.6703053
Oliver-Fernandez A, Bakal J, Segal S, Shah GK, Dugar A, Sharma S (2005) Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Can J Ophthalmol 40:313–319. doi:10.1139/i05-003
Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I (2008) The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 115:116–126. doi:10.1016/j.ophtha.2007.03.008
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N (2008) Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 145:239–248. doi:10.1016/j.ajo.2007.10.004
Acknowledgements
This study was supported by the Koeln Fortune Program / Faculty of Medicine, University of Cologne.
The authors thank Dr. Derek J. Saunders for critical reading and editing of the manuscript. The authors also thank Susanne Nelles for database maintenance.
Financial interest
None for all authors
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne
The authors have full control of all primary data and agree to allow Graefes Archive for Clinical and Experimental Ophthalmology to review the data on request.
Rights and permissions
About this article
Cite this article
Muether, P.S., Hermann, M.M., Koch, K. et al. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefes Arch Clin Exp Ophthalmol 249, 633–637 (2011). https://doi.org/10.1007/s00417-010-1520-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1520-9