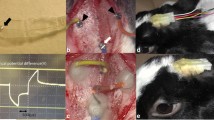
Abstract
Background
To determine the efficient parameters to evoke electrical phosphenes is essential for the development of a retinal prosthesis. We studied the efficient parameters in normal subjects and investigated if suprachoroidal-transretinal stimulation (STS) is effective in patients with advanced retinitis pigmentosa (RP) using these efficient parameters.
Methods
The amplitude of pupillary reflex (PR) evoked by transcorneal electrical stimulation (TcES) was determined at different frequencies in eight normal subjects. The relationship between localized phosphenes elicited by transscleral electrical stimulation (TsES) and the pulse parameters was also examined in six normal subjects. The phosphenes evoked by STS were examined in two patients with RP with bare light perception. Biphasic pulses (cathodic first, duration: 0.5 or 1.0 ms, frequency: 20 Hz) were applied through selected channel(s). The size and shape of the phosphenes perceived by the patients were recorded.
Results
The maximum PR was evoked by TcES with a frequency of 20 Hz. The brightest phosphene was elicited by TsES with a pulse train of more than 10 pulses, duration of 0.5–1.0 ms and a frequency of 20 to 50 Hz. In RP patients, localized phosphenes were elicited with a current of 0.3–0.5 mA (0.5 ms) in patient 1 and 0.4 mA (1.0 ms) in patient 2. Two isolated or dumbbell-shaped phosphenes were perceived when the stimulus was delivered through two adjacent channels.
Conclusion
Biphasic pulse trains (≥10 pulses) with a duration of 0.5–1.0 ms and a frequency of 20–50 Hz were efficient for evoking phosphenes by localized extraocular stimulation in normal subjects. With these parameters, STS is a feasible method to use with a retinal prosthesis even in advanced stages of RPs.








Similar content being viewed by others
References
Chow AY, Chow VY (1997) Subretinal electrical stimulation of the rabbit retina. Neurosci Lett 225:13–16
Chow AY, Chow VY, Packo KH, Pollack JS, Peyman GA, Schuchard R (2004) The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol 122:460–469
Chowdhury V, Morley JW, Coroneo AM (2005) Stimulation of the retina with a multiekectrode extraocular visual prosthesis. ANZ J Surg 75:679–704
Hesse L, Schanze T, Wilms M, Eger M (2000) Implantation of retina stimulation electrodes and recording of electrical stimulation responses in the visual cortex of the cat. Graefe Arch Clin Exp Ophthalmol 238:840–845
Humayun MS, Price M, de Juan E Jr et al (1999) Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci 40:143–148
Humayun MS, de Juan E, Weiland JD, Dagnelie G, Katona S, Greenberg R, Suzuki S (1999) Pattern electrical stimulation of human retina. Vision Res 39:2569–2576
Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G, de Juan E (2003) Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res 43:2573–2581
Jensen RJ, Rizzo JF 3rd, Ziv OR, Grumet A, Wyatt J (2003) Thresholds for activation of rabbit retinal ganglion cells with an ultrafine, extracellular microelectrode. Invest Ophthalmol Vis Sci 44:3533–3543
Jensen RJ, Ziv OR, Rizzo JF 3rd (2005) Thresholds for activation of rabbit retinal ganglion cells with relatively large, extreacellular microelectrodes. Invest Ophthalmol Vis Sci 46:1486–1496
Kanda H, Morimoto T, Fujikado T, Tano Y, Fukuda Y, Sawai H (2004) Electrophysiological studies on the feasibility of suprachoroidal-transretinal stimulation for artificial vision in normal and RCS Rat. Invest Ophthalmol Vis Sci 45:560–566
Kawasumi M (1981) Distribution of current intensities inside the electrically stimulated eye. Nippon Ganka Gakkai Zasshi 89:766–772
Leventhal AG, Rodieck RW, Dreher B (1981) Retinal ganglion cell classes in the old world monkey: morphology and central projections. Science 213:1139–1142
Majji AB, Humayun MS, Weiland JD, Suzuki S, D’Anna SA, de Juan E Jr (1999) Long-term histological and electrophysiological results of an inactive epiretinal electrode array implantation in dogs. Invest Ophthalmol Vis Sci 40:2073–2081
Margalit E, Maia M, Weiland JD, Greenberg RJ, Fujii GY, Torres G, Piyathaisere DV, O’Hearn TM, Liu W, Lazzi G, Dagnelie G, Scribner DA, de Juan E Jr, Humayun MS (2002) Retinal prosthesis for the blind. Surv Ophthalmol 47:335–356
Marmor MF, Aguirre G, Arden G et al (1983) Retinitis pigmentosa: a symposium on terminology and methods of examination. Ophthalmology 90:126–131
Miyake Y, Yanagida K, Yagasaki K (1980) Clinical application of EER (electrically evoked response) (2) Analysis of EER in patients with dysfunctional rod or cone visual pathway. Nippon Ganka Gakkai Zasshi 84:502–509
Morimoto T, Fukui T, Matsushita K, Okawa Y, Shimojyo H, Kusaka S, Tano Y, Fujikado T (2006) Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefe Arch Clin Exp Ophthalmol 244:1283–1292
Motokawa K, Ebe M (1952) Selective stimulation of color receptors with alternating currents. Science 25(115):92–94
Nakauchi K, Fujikado T, Kanda H, Morimoto T, Choi JS, Ikuno Y, Sakaguchi H, Kamei M, Ohji M, Yagi T, Nishimura S, Sawai H, Fukuda Y, Tano Y (2005) Transretinal electrical stimulation by an intrascleral multichannel electrode array in rabbit eyes. Graefe Arch Clin Exp Ophthalmol 243:169–174
Pagon RA (1988) Retinitis pigmentosa. Surv Ophthalmol 33:137–177
Potts AM, Inoue J (1969) The electrically evoked response of the visual system (EER) II. Effect of adaptation and retinitis pigmentosa. Invest Ophthalmol 8:605–613
Rizzo JF 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D (2003) Method and perceptual threshold for short-term electrical stimulation of human retina with a microelectrode array. Invest Ophthalmol Vis Sci 44:5355–5361
Rizzo JF 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D (2003) Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest Ophthalmol Vis Sci 44:5362–5369
Santos A, Humayun MS, de Juan E Jr, Greenburg RJ, Marsh MJ, Klock IB, Milam AH (1997) Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Arch Ophthalmol 115:511–515
Schwahn HN, Gekeler F, Kohler K, Kobuch K, Sachs HG, Schulmeyer F, Jakob W, Gabel VP, Zrenner E, Schwahn HN (2001) Studies on the feasibility of a subretinal visual prosthesis: data from Yucatan micropig and rabbit. Graefe Arch Clin Exp Ophthalmol 239:961–967
Stone J, Fukuda Y (1974) Properties of cat’s retinal ganglion cells: a comparison of W-cells with X-cells and Y-cells. J Neurophysiol 37:722–748
Stone JL, Barlow WE, Humayun MS, de Juan E Jr, Milam AH (1992) Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol 110:1634–1639
Tanino T, Kato S, Kawasumi M (1981) Studies on electrically evoked pupillary reflex-Indirect reflex and its frequency characteristics. Jpn J Ophthalmol 25:423–429
Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini S, Wanet-Defalque MC (1998) Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res 813:181–186
Walter P, Heimann K (2000) Evoked cortical potentials after electrical stimulation of the inner retina in rabbits. Graefe Arch Clin Exp Ophthalmol 238:315–318
Zrenner E (2002) Will retinal implants restore vision? Science 295:1022–1025
Acknowledgements
The authors thank Yozo Miyake, Satoshi Suzuki, Mineo Kondo Yutaka Fukuda, Hajime Sawai and Tomomitsu Miyoshi for advice and discussion.
Commercial interest
Hiroyuki Kanda and Motoki Ozawa are employees of the Nidek Company.
Financial support
This study was supported by Health Sciences Research Grants (H16-sensory-001) from the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujikado, T., Morimoto, T., Kanda, H. et al. Evaluation of phosphenes elicited by extraocular stimulation in normals and by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 245, 1411–1419 (2007). https://doi.org/10.1007/s00417-007-0563-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0563-z