Abstract
Purpose
To evaluate the effect of hexanic extract of Serenoa repens (HESr) on prostatic inflammation in patients with diagnosed prostatic inflammation.
Methods
Patients with prostatic inflammation histologically confirmed by TRUS prostatic biopsy were randomized either to receive HESr (320 mg/day) or no treatment. A second biopsy was performed 6 months later according to standard clinical practice. Inflammation was assessed by the Irani’s score and immunohistochemical staining using the CD3, CD4 and CD8 (for T-leucocytes), CD20 (for B-leucocytes) and CD163 (for macrophages) antibodies.
Results
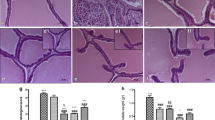
Overall 97 patients were eligible for analysis. In the HESr group the mean inflammation grading and aggressiveness grading score significantly decreased from 1.55 and 1.55 at baseline to 0.79 (p = 0.001) and 0.87 (p = 0.001) at the second biopsy, respectively. In the control group the mean inflammation grading score was 1.44 at first biopsy and 1.23 at the second biopsy. The mean aggressiveness gradings core was 1.09 and 0.89, respectively. No statistical significance was found (p = 0.09 and p = 0.74).The mean decrease in all inflammation scores was statistically higher in the HESr patients compared to controls. The immunohistochemical staining showed a significant change in the expression of the analyzed antibodies for the HESr patients compared to the first biopsy. In the nontreatment group, no significant difference was found at the second biopsy. The change in expression of each antibody in the HESr group was statistical significant compared to control.
Conclusions
HESr seems to reduce prostatic inflammation in terms of histological and immunohistochemical parameters in this specific patients population.


Similar content being viewed by others
References
Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS (2008) The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 54(6):1379–1384
De Nunzio C, Kramer G, Marberger M et al (2011) The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 60:106–117
Di Silverio F, Gentile V, De Matteis A et al (2003) Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol 43:164–175
Samarinas M, Gacci M, de la Taille A, Gravas S (2018) Prostatic inflammation: a potential treatment target for male LUTS due to benign prostatic obstruction. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/s41391-018-0039-8
Buck AC (2004) Is there a scientific basis for the therapeutic effects of Serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol 172:1792–1799
De la Taille A (2013) Therapeutic approach: the importance of controlling prostatic inflammation. Eur Urol Suppl 12:116–122
Vela Navarrete R, Garcia Cardoso JV, Barat A, Manzarbeitia F, López Farré A (2003) BPH and inflammation: pharmacological effects of permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur Urol 44:549–555
Latil A, Pétrissans MT, Rouquet J et al (2015) Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 75(16):1857–1867
Irani J, Levillain P, Goujon JM, Bon D, Dore B, Aubert J (1997) Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol 157(4):1301–1303
Robert G, Descazeaud A, Nicolaïew N, Terry S, Sirab N, Vacherot F, Maillé P, Allory Y, de la Taille A (2009) Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate 69(16):1774–1780
Novara G, Giannarini G, Alcaraz A et al (2016) Efficacy and safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. Eur Urol Focus 2(5):553–561
Vela-Navarrete R, Alcaraz A, Rodríguez-Antolín A et al (2018) Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): systematic review and meta-analysis of randomized controlled trials and observational studies. BJU Int. https://doi.org/10.1111/bju.14362 (in press)
Author information
Authors and Affiliations
Contributions
SG: protocol and project development, data collection, data analysis, manuscript editing. MS: data collection, data analysis, manuscript writing. KZ: data collection and management. AK: data collection. GK: data collection and management, manuscript editing. VT: data collection. MM: data collection and management, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
Stavros Gravas has received speaker honoraria from Astellas, Pierre Fabre Medicament and consultancy honoraria from Astellas and GSK. Michael Samarinas, Konstantina Zacharouli, Anastasios Karatzas, Georgios Koukoulis, Vasileios Tzortzis, Michael Melekos declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Gravas, S., Samarinas, M., Zacharouli, K. et al. The effect of hexanic extract of Serenoa repens on prostatic inflammation: results from a randomized biopsy study. World J Urol 37, 539–544 (2019). https://doi.org/10.1007/s00345-018-2409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2409-1