Abstract
Purpose
To evaluate the efficacy and safety of adding a low-dose oral desmopressin to tamsulosin therapy for treatment of nocturia in patients with benign prostatic hyperplasia (BPH).
Methods
Eligible patients with BPH and nocturia ≥2/night were randomly allocated to two treatment groups; the first of which received 3-month treatment scheme of daily oral dose of tamsulosin OCAS 0.4 mg and desmopressin MELT 60 mcg (D/T group), while the second one received tamsulosin OCAS 0.4 mg only (T group). Patients were followed on monthly basis and changes in the parameters from baseline to 3 months after treatment were assessed on I-PSS/QoL questionnaire, 7-day voiding diary, urinalysis, serum sodium, abdominal ultrasonography and uroflowmetry.
Results
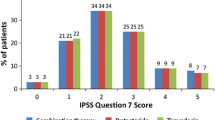
A total of 248 patients were included within the study; 123 patients in the combined D/T group and 125 patients in T group. The frequencies of night voids decreased by 64.3 % in D/T group compared to 44.6 % in T group. The first sleep period, significantly increased from 82.1 to 160.0 min and from 83.2 to 123.8 min in D/T and T group, respectively; and significant differences between both groups were observed at the end of study (p < 0.001). I-PSS, QoL score, post-void residual urine volume and Q max were significantly improved with no statistical difference between both groups. No serious adverse effects were reported in both groups.
Conclusion
The addition of low-dose oral desmopressin therapy to an α-blocker tamsulosin provides effective treatment for nocturia in patients with LUTS/BPH.


Similar content being viewed by others
References
van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, Jennum P, Johnson T, Lose G, Mattiasson A, Robertson G, Weiss J (2002) The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21(2):179–183
Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK (2009) Nocturia and disturbed sleep in the elderly. Sleep Med 10(5):540–548
Tikkinen KA, Johnson TM II, Tammela TL, Sintonen H, Haukka J, Huhtala H, Auvinen A (2010) Nocturia frequency, bother, and quality of life: How often is too often? A population-based study in Finland. Eur Urol 57(3):488–496
Yoshimura K, Ohara H, Ichioka K, Terada N, Matsui Y, Terai A, Arai Y (2003) Nocturia and benign prostatic hyperplasia. Urology 61(4):786–790
van Kerrebroeck P, Rezapour M, Cortesse A, Thuroff J, Riis A, Norgaard JP (2007) Desmopressin in the treatment of nocturia: a double-blind, placebo-controlled study. Eur Urol 52(1):221–229
Montorsi F, Mercadante D (2013) Diagnosis of BPH and treatment of LUTS among GPs: a European survey. Int J Clin Pract 67(2):114–119
Wang CJ, Lin YN, Huang SW, Chang CH (2011) Low dose oral desmopressin for nocturnal polyuria in patients with benign prostatic hyperplasia: a double-blind, placebo controlled, randomized study. J Urol 185(1):219–223
Kobelt G, Borgstrom F, Mattiasson A (2003) Productivity, vitality and utility in a group of healthy professionally active individuals with nocturia. BJU Int 91(3):190–195
Van Dijk MM, Wijkstra H, Debruyne FM, De La Rosette JJ, Michel MC (2010) The role of nocturia in the quality of life of men with lower urinary tract symptoms. BJU Int 105(8):1141–1146
Kim JJ, Han DH, Sung HH, Choo SH, Lee SW (2014) Efficacy and tolerability of tamsulosin 0.4 mg in Asian patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia refractory to tamsulosin 0.2 mg: a randomized placebo controlled trial. Int J Urol 21(7):677–682
Schwinn DA, Roehrborn CG (2008) Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol 15(3):193–199
Abrams P (2012) Re: Update on AUA guideline on the management of benign prostatic hyperplasia: KT McVary, CG Roehrborn, AL Avins, MJ Barry, RC Bruskewitz, RF Donnell, HE Foster, Jr., CM Gonzalez, SA Kaplan, DF Penson, JC Ulchaker and JT Wei. J Urol 2011; 185:1793–1803. J Urol;187(1):358-9; author reply 9
Spearkman M (2006) Efficacy and safety of tamsulosin OCAS. BJU Int 98(suppl. 2):13–17
Smith AL, Wein AJ (2011) Outcomes of pharmacological management of nocturia with non-antidiuretic agents: does statistically significant equal clinically significant? BJU Int 107(10):1550–1554
Johnson TM 2nd, Burrows PK, Kusek JW, Nyberg LM, Tenover JL, Lepor H, Roehrborn CG (2007) The effect of doxazosin, finasteride and combination therapy on nocturia in men with benign prostatic hyperplasia. J Urol 178(5):2045–2050
Schneider T, de la Rosette JJ, Michel MC (2009) Nocturia: a non-specific but important symptom of urological disease. Int J Urol 16(3):249–256
Weiss JP, Weinberg AC, Blaivas JG (2008) New aspects of the classification of nocturia. Curr Urol Rep 9(5):362–367
Chang SC, Lin AT, Chen KK, Chang LS (2006) Multifactorial nature of male nocturia. Urology 67(3):541–544
Vilhardt H (1990) Basic pharmacology of desmopressin. Rev Drug Invest 2:28
Ahmed AF, Amin MM, Ali MM, Shalaby EA (2013) Efficacy of an enuresis alarm, desmopressin, and combination therapy in the treatment of Saudi children with primary monosymptomatic nocturnal enuresis. Korean J Urol 54(11):783–790
Naghizadeh S, Kefi A, Dogan HS, Burgu B, Akdogan B, Tekgul S (2005) Effectiveness of oral desmopressin therapy in posterior urethral valve patients with polyuria and detection of factors affecting the therapy. Eur Urol 48(5):819–825
Koca OKM, Güneş M, Öztürk M, Akyüz M (2012) Karaman Mİ Desmopressin in the treatment of nocturia with BPH. Turk J Urol 1(28):29–31
Addla SK, Adeyoju AB, Neilson D, O’Reilly P (2006) Diclofenac for treatment of nocturia caused by nocturnal polyuria: a prospective, randomised, double-blind, placebo-controlled crossover study. Eur Urol 49(4):720–725
Araki T, Yokoyama T, Kumon H (2004) Effectiveness of a nonsteroidal anti-inflammatory drug for nocturia on patients with benign prostatic hyperplasia: a prospective non-randomized study of loxoprofen sodium 60 mg once daily before sleeping. Acta Med Okayama 58(1):45–49
Bae WJ, Bae JH, Kim SW, Chung BH, Kim JH, Kim CS, Lee HM, Lee KS, Yoo TK, Kim SI, Byun SS, Lee JY (2013) Desmopressin add-on therapy for refractory nocturia in men receiving alpha-blockers for lower urinary tract symptoms. J Urol 190(1):180–186
Yoshida M, Inadome A, Masunaga K, Nagata T, Yoshiyasu T (2010) Effectiveness of tamsulosin hydrochloride and its mechanism in improving nocturia associated with lower urinary tract symptoms/benign prostatic hyperplasia. Neurourol Urodyn 29(7):1276–1281
Delfanian K, Zawada ET Jr (2001) DDAVP-associated hyponatremia. S D J Med 54(7):255–256
Acknowledgments
The authors are grateful to Margie A. Briones, Elizabith C. Arrieta, Swapna C. Muriankarry and Jissy T. Madakkampurath for technical assistance and reporting data.
Conflict of interest
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Ethical standard
Based on the protocol that was reviewed and approved by the institutional ethics committee of the participating institutions, the study procedure was explained in detail to all patients and each patient provided a written informed consent before enrollment; the patients had full right to withdraw from the study at any time, and all patients’ data were coded by study identification number.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, Af., Maarouf, A., Shalaby, E. et al. The impact of adding low-dose oral desmopressin therapy to tamsulosin therapy for treatment of nocturia owing to benign prostatic hyperplasia. World J Urol 33, 649–657 (2015). https://doi.org/10.1007/s00345-014-1378-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1378-2