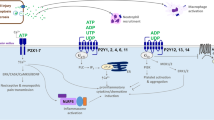
Abstract
Purpose
Dysregulation of neurotransmitter receptors may contribute to bladder overactivity (OAB) symptoms. To address the question whether specific receptor expression patterns are associated with bladder pain syndrome/interstitial cystitis (BPS/IC), we examined the expression of muscarinic, purinergic and histamine receptors in the detrusor.
Methods
Detrusor receptor expression was investigated in bladder biopsies of female BPS/IC patients (n = 44; age 60.64 ± 13.78, mean ± SD) and carcinoma patients (n = 11; age 58.91 ± 12.72) undergoing cystectomy. Protein expression of muscarinic (M2, M3), purinergic (P2X1–3) and histamine receptors (H1, H2) was analysed by confocal immunofluorescence, and gene expression was quantified by real-time polymerase chain reaction (qPCR).
Results
M2, P2X1, P2X2 and H1 receptor immunoreactivity (-IR) was significantly enhanced in BPS/IC compared to the control group, while there was no difference for M3-, P2X3- and H2-IR. We calculated a score, which separated BPS/IC from control patients with an AUC of 89.46%, showing 84.09% sensitivity and 90.91% specificity. Patients had a 9.25 times enhanced calculated risk for BPS/IC. In addition, two patient subgroups (M2 > M3 and M3 > M2) were observed, which differed in associated purinergic and histamine receptor expression.
Conclusions
M2, P2X1, P2X2 and H1 were significantly upregulated in BPS/IC patients, and H2 was occasionally highly overexpressed. There was no significant correlation between receptor protein and gene expression, implying posttranslational mechanisms being responsible for the altered receptor expressions. On the basis of individual receptor profiles, upregulated receptors could be targeted by monotherapy or combination therapy with already approved receptor inhibitors, thereby promoting tailored therapy for patients suffering from BPS/IC-like symptoms.


Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49
Parsons JK, Parsons CL (2004) The historical origins of interstitial cystitis. J Urol 171:20–22
Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg LJ (1999) The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol 161:553–557
van de Merwe JP, Nordling J, Bouchelouche P et al (2008) Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 53:60–67
Fall M, Baranowski AP, Elneil S et al (2010) EAU guidelines on chronic pelvic pain. Eur Urol 57:35–48
Parsons CL (2007) The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69:9–16
Nazif O, Teichman JM, Gebhart GF (2007) Neural upregulation in interstitial cystitis. Urology 69:24–33
Erickson DR (2001) Urine markers of interstitial cystitis. Urology 57:15–21
Neuhaus J, Pfeiffer F, Wolburg H, Horn LC, Dorschner W (2005) Alterations in connexin expression in the bladder of patients with urge symptoms. BJU Int 96:670–676
Mukerji G, Yiangou Y, Grogono J et al (2006) Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol 176:367–373
Pontari MA, Braverman AS, Ruggieri MRS (2004) The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am J Physiol Regul Integr Comp Physiol 286:R874–R880
Frazier EP, Peters SL, Braverman AS, Ruggieri MRS, Michel MC (2008) Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 377:449–462
Ford AP, Gever JR, Nunn PA et al (2006) Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147:S132–S143
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23
Sun Y, Chai TC (2006) Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290:C27–C34
Sant GR, Kempuraj D, Marchand JE, Theoharides TC (2007) The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 69:34–40
Neuhaus J, Oberbach A, Schwalenberg T, Stolzenburg JU (2006) Cultured smooth muscle cells of the human vesical sphincter are more sensitive to histamine than are detrusor smooth muscle cells. Urology 67:1086–1092
Bouchelouche K, Bouchelouche P (2006) Cysteinyl leukotriene d(4) increases human detrusor muscle responsiveness to histamine. J Urol 176:361–366
Thilagarajah R, Witherow RO, Walker MM (2001) Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int 87:207–212
Theoharides TC, Sant GR (1997) Hydroxyzine therapy for interstitial cystitis. Urology 49:108–110
Hanno PM, Burks DA, Clemens JQ et al (2011) AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 185:2162–2170
Rasband WS ImageJ. US National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2006
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Varambally S, Yu J, Laxman B et al (2005) Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 8:393–406
Mansfield KJ, Liu L, Moore KH, Vaux KJ, Millard RJ, Burcher E (2007) Molecular characterization of M2 and M3 muscarinic receptor expression in bladder from women with refractory idiopathic detrusor overactivity. BJU Int 99:1433–1438
Ruggieri MRS, Braverman AS (2006) Regulation of bladder muscarinic receptor subtypes by experimental pathologies. Auton Autacoid Pharmacol 26:311–325
Braverman AS, Tibb AS, Ruggieri MRS (2006) M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther 316:869–874
Acknowledgment
We thank Mrs. A. Weimann, Mrs. M. Berndt and Mrs. K. Büttner for their excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
345_2011_774_MOESM1_ESM.doc
Table S1: Confocal immunofluorescence analysis results. Numbers represent fluorescence intensities of the receptor stainings normalized to background fluorescence (FIrec/FIbkgd). (DOC 41 kb)
345_2011_774_MOESM2_ESM.doc
Table S2: Correlation analysis. Nonparametric correlation coefficient (Spearman r) is reported. Except for P2RX1 receptor, there was no significant correlation between receptor gene expression and protein expression. (DOC 32 kb)
345_2011_774_MOESM3_ESM.doc
Table S3: Correlation analysis. Nonparametric correlation coefficient (Spearman r) is reported. There was no relevant correlation of receptor expression with age in either group. Note the high Spearman r for CHRM2 and P2RX1 with R_Score, indicating major contribution of those receptors. * p < 0.05; ** p < 0.01; *** p < 0.001; na = not applicable. (DOC 59 kb)
345_2011_774_MOESM4_ESM.doc
Table S4: Correlation between receptor immunofluorescence in SM2 and SM3 patients. 82% of the patients showed M2 > M3 expression (SM2). Note the high number of significant correlations between receptors (Spearman r, *p < 0.05). 18% of the patients showed M3 > M2 expression (SM3) with only few significant correlations of receptor expression. * p < 0.05; ** p < 0.01; *** p < 0.001. (DOC 53 kb)
345_2011_774_MOESM5_ESM.tif
Figure S1: Workflow of confocal immunofluorescence quantitative analysis. (a) Confocal image of staining control (StainCo); (b) M2 receptor staining; regions of interest (ROIs) marked. (c-d) Pixel intensity distribution is measured in 35 ROIs, defined as alpha-smooth muscle cell actin-positive areas. Nuclei were excluded from analysis. (e) Pixel distribution normalized to StainCo (FIRec/FIStainCo). Note the excellent signal to noise ratio as demonstrated by clear separation of the M2 receptor staining (clear area) and staining control (dark grey). (f) Receptor profile including statistical analysis by ANOVA with Tukey’s post hoc test; bars: p < 0.05 was considered significant; § indicates no significant receptor expression. (TIFF 3212 kb)
345_2011_774_MOESM6_ESM.tif
Figure S2: Assessment of antibody specificity. Antibody specificity was tested by comparison of confocal immunofluorescence intensities of sections stained with specific receptor antibodies and corresponding sections where the peptide, to which the antibody was raised, was used to block the antibody-antigen detection. The peptides were purchased from the supplier of the corresponding antibody. The samples were processed and analysed according to the standard protocol. (a) Quantitative analysis revealed significant diminution of the fluorescence intensity by blocking peptide (dilution 1:100–1:2000). (b) Representative receptor immunofluorescence confocal images. (TIFF 5101 kb)
Rights and permissions
About this article
Cite this article
Neuhaus, J., Schulte-Baukloh, H., Stolzenburg, JU. et al. Individual receptor profiling as a novel tool to support diagnosis of bladder pain syndrome/interstitial cystitis (BPS/IC). World J Urol 30, 693–700 (2012). https://doi.org/10.1007/s00345-011-0774-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-011-0774-0