Abstract
Objective
We investigated the feasibility of serial dynamic contrast-enhanced computed tomography (DCE-CT) in patients with advanced/metastatic non-small cell lung cancer (NSCLC) receiving anti-angiogenic (sorafenib) and anti-EGFR (erlotinib) treatment, and correlated tumour blood flow (BF) with treatment outcome.
Methods
DCE-CTs were performed at baseline and 3 and 6 weeks after starting treatment. Tumour BF, calculated with the maximum slope method, and percentage change were measured in 23 patients (14 male; median age 59 years). Tumour BF was compared at baseline and weeks 3 and 6; the relation with RECIST/Crabb response and progression-free survival (PFS) was assessed.
Results
Mean tumour perfusion decreased from 39.2 ml/100 g/min at baseline to 15.1 ml/100 g/min at week 3 (p < 0.001) and 9.4 ml/100 g/min at week 6 (p < 0.001). Tumour perfusion was lower in RECIST and Crabb responders versus non-responders at week 3 (4.2 versus 17.7 ml/100 g/min, p = 0.03) and week 6 (0 versus 13.4 ml/100 g/min, p = 0.04). Patients with a decrease larger than the median at week 6 tended to have a longer PFS (7.1 versus 5.7 months, p = 0.06).
Conclusion
Serial DCE-CTs are feasible in patients with NSCLC and demonstrated a significant decrease in tumour BF following sorafenib/erlotinib therapy. Early changes in tumour BF correlated with objective response and showed a trend towards longer PFS.
Similar content being viewed by others
Introduction
Angiogenesis, the formation of new blood vessels, is a key process in tumorigenesis [1]. Angiogenesis inhibitors have yielded promising results in oncology. They are considered largely cytostatic, inhibiting tumour growth and progression rather than causing tumour regression. Furthermore, anatomical changes lag behind functional changes and tumour shrinkage does not necessarily correlate with patient survival [2]. The standard anatomy-based response assessment using the Response Evaluation Criteria in Solid Tumors (RECIST), therefore seems inadequate for the early evaluation of the efficacy of these drugs [3, 4].
A logical approach to evaluating the effect of angiogenesis inhibitors is to assess their proposed direct target: the tumour vasculature. The current gold standard technique to determine tumour vascularity is measurement of microvessel density (MVD) on tissue samples [5]. However, this invasive method is unsuitable for serial measurements. Furthermore, only focal regions are assessed whereas tumour vasculature is heterogeneous. Non-invasive functional imaging techniques may be superior, providing information on whole tumour volumes. Traditionally dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has been used. However, its application in lung cancer is limited by a low spatial resolution. With the development of multi-detector CT and user-friendly perfusion software programs, the quantification of perfusion parameters using dynamic contrast-enhanced CT (DCE-CT) is increasingly being investigated in oncology.
Currently, there are limited data available concerning the application of DCE-CT for response monitoring of angiogenesis inhibitors. However, this does appear to be feasible and changes in tumour vascular parameters have been demonstrated [6–10]. In non-small cell lung cancer (NSCLC) whole tumour perfusion measured by DCE-CT has been shown to be reproducible [11, 12]. Perfusion parameters measured before surgery for lung cancer correlate with MVD in resected tumour specimens, implying a reliable assessment of tumour vascularity [13, 14]. No studies have, to our knowledge, assessed DCE-CT in monitoring response to angiogenesis inhibitors in NSCLC and how this correlates with patient outcome [15].
In the context of a phase II trial we prospectively evaluated the tumour blood flow (BF) measured by DCE-CT in patients with advanced or metastatic NSCLC treated with the multi-targeted tyrosine kinase angiogenesis inhibitor sorafenib and the anti-epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor erlotinib. Both have demonstrated anti-tumour efficacy in NSCLC [16–19]. The study aimed to investigate the feasibility of serial DCE-CTs in NSCLC patients receiving combined anti-angiogenic and anti-EGFR treatment, to determine the effect of sorafenib plus erlotinib on tumour BF and to relate tumour BF to treatment outcome.
Materials and methods
Patient selection
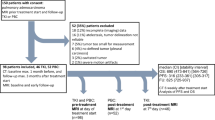
The study was conducted within a multi-centre phase II clinical trial of erlotinib and sorafenib in chemotherapy-naïve patients with inoperable, locally advanced/metastatic NSCLC (NCT00722969). Two out of the three participating centres took part in this side study. Approval was obtained from each centre’s institutional review board. All patients provided written informed consent.
Between December 2007 and October 2008, 34 of the 50 patients in the phase II trial were prospectively enrolled onto this study. Inclusion criteria included Eastern Cooperative Oncology Group performance status 0–1, estimated life expectancy ≥ 12 weeks and primary tumour size ≥ 1 cm on CT. Patients with a known contrast medium allergy, the inability to hold their breath for long enough or the inability to obey breath-hold commands were ineligible.
Study design
Erlotinib (150 mg/day) and sorafenib (800 mg/day) were orally self-administered and continued until disease progression, unacceptable toxicity and/or withdrawal of consent. Dose reductions were allowed but did not occur within the first 3 weeks of treatment. Clinical assessments were scheduled every 3 weeks for the first 3 months then every 6 weeks. DCE-CT was performed at the same time as pre-treatment CTs and 3 and 6 weeks after starting treatment. Tumour anatomic size was evaluated at baseline, weeks 3 and 6 and then 6-weekly by standard contrast-enhanced CT of the thorax. Tumour anatomic response was assessed by change in size according to RECIST 1.0 and an alternative method incorporating tumour cavitation as proposed by Crabb et al. (a target lesion is measured by subtracting the longest diameter of a cavity [zero if no cavity present] from the longest diameter of the lesion) [20].
DCE-CT acquisition protocol
The DCE-CTs were obtained by using 64-slice multi-detector CT (MDCT) (Somatom Sensation, Siemens, Erlangen, Germany). An initial non-enhanced CT study (80 kV, 120 mAs , collimation 24 × 1.2 mm, pitch 1.15, rotation time 0.5 s, field of view 380 mm, slice width 5 mm, reconstruction increment 5 mm) was performed to determine the position of the primary tumour. The subsequent dynamic spiral CT series encompassed the whole primary tumour volume. A 50-ml intravenous bolus of a low-osmolar non-ionic contrast agent with an iodine concentration of 300 mg/ml (Ultravist-300 Iopromide; Schering A.G., Berlin, Germany) was injected over 6.25 s (8 ml/s), using an injection pump through an 18-gauge needle. After a start delay of 4 s, dynamic acquisition of image data was obtained over approximately 40 s, within one breath-hold period. CT parameters were 100 kV and 80 mAs at a slice collimation of 64 × 0.6 mm and a rotation time of 0.5 s. Temporal resolution was 4 s (1-s acquisition time; interscan delay 3 s). The images were reconstructed by using a slice thickness of 5 mm, with a reconstruction increment of 5 mm. Dynamic CTs were performed with a fixed lower tube current of 80 mAs to reduce radiation exposure. After this dynamic acquisition and following an additional 80-ml bolus of the same low-osmolar non-ionic contrast agent at an injection rate of 3 ml/s and a 30-s delay, a routine, post-contrast, spiral CT of the thorax (120 kV; 130 mAs with care dose automated exposure control) was obtained by using 5-mm-thick sections with a contiguous reconstruction algorithm. For a z-axis coverage of 5 cm (which is variable and depends on tumour size) the effective dose, expressed according to the International Commission on Radiological Protection recommendations, was 1.2 mSv for the non-enhanced CT and 4.4 mSv (10 × 0.44 mSv) for the dynamic CT protocol [21–23].
Post-processing analysis
Image registration (3D rigid-body) was performed with a commercially available 3D image fusion program (Vinci 2.36.0, Max-Planck Institute for Neurological Research, Cologne). For the quantification of tissue perfusion and for the creation of blood flow maps, the software program Basama Perfusion 3.0.7.1 (Kanazawa, Ishikawa, Japan) was used [24, 25]. This program uses the maximum slope method, calculating perfusion as the maximum slope of the tumour time–density curve divided by the peak arterial enhancement [26]:
where F is blood flow, V is blood volume, c(t) is the contrast density in the tissues and a(t) is the contrast density in the feeding artery (aorta). Regions of interest (ROIs) were drawn within the aorta and within the tumour (as large as possible to minimise noise but excluding large cavitations and extensive areas of necrosis [density < 5 HU]) (see Fig. 1). Tumour ROIs were defined in all slices that covered the tumour and the tumour BF was calculated as follows:
An example of serial DCE-CT and perfusion parameter maps of the thorax (axial plane) of a study patient. a Tumour blood flow at baseline was 95.2 ml/100 g/min. b After 3 weeks of treatment tumour cavitation was present and the blood flow had decreased to 18.2 ml/100 g/min. c After 6 weeks there was more extensive tumour cavitation. Blood flow in the remaining peripheral tumour tissue had decreased to 7.0 ml/100 g/min
Blood flow is expressed in millilitres per 100g of tumour tissue per minute (ml/100 g/min). Imaging analysis was performed by an experienced radiologist blinded to the clinical outcome.
Statistical analysis
Baseline (pre-treatment) tumour BF was compared with tumour BF after 3 and 6 weeks of treatment using the non-parametric Wilcoxon signed-rank test. Tumour BF and percentage change in tumour BF were compared between responders (defined as those achieving an objective complete or partial response [PR] according to the RECIST and Crabb methods) versus non-responders (defined as those having stable disease [SD] or progressive disease [PD]) using the Mann–Whitney test. Log-rank statistics were used to test associations with progression-free survival (PFS) and overall survival (OS). Two-tailed p < 0.05 was considered statistically significant.
Results
The study protocol was easily integrated into the clinical routine. No side effects were seen and all patients tolerated the dynamic CT well. The clinical characteristics of the 34 patients included did not differ from the entire study population of the clinical trial (Table 1). Thirty patients had at least two DCE-CTs. Reasons for less than two CT being performed were: logistical problems (n = 2); discontinuation of study medication (n = 1) and withdrawal of consent (n = 1). Seven patients were excluded from the perfusion analysis. Reasons for exclusion were: inclusion errors (tumour too small [n = 3]; tumour not evaluable due to post-obstructive atelectasis and a large pleural effusion [n = 1]); beam-hardening artefact due to the close proximity of the tumour to a major blood vessel (n = 2); and technical imaging error (n = 1). Of the 23 patients included in the analysis 19 had tumour BF measurements at all three time points. There were 14 male and 9 female patients with a median age of 59 years (range 41–78 years). Five patients had stage IIIB and 18 stage IV (Table 1). After a median follow-up of 10.9 months (95% CI 9.2–12.7) 12 patients remain alive of whom 9 are progression-free. Median PFS was 5.9 months (95% CI 5.4–6.4) and median OS 12.4 months (95% CI 10.3–14.6), which is similar to the total clinical trial patient population (PFS 4.6 months and OS 12.0 months) (Table 1).
Anatomical response evaluation
Mean tumour size decreased from 5.6 cm (range 1.9–14.0 cm) at baseline to 4.8 cm (range 1.6–11.0 cm) at week 3 and 4.6 cm (range 1.4–10.1 cm) at week 6. The mean change in size was −13% (range −45 to +5%) and −19% (range −38 to 0%) respectively. The RECIST response was unavailable for 2 out of the 23 patients: one patient discontinued treatment before the week 6 response CT and one patient was not evaluable for response because of the development of a large cavity superimposed with infection. Of the evaluable patients, 6 had PR and 15 SD as their best objective primary tumour response according to RECIST (Table 1). Nine patients developed extensive cavitations during treatment. No tumour cavitation was observed at baseline. According to the Crabb criteria 11 patients had PR and 10 SD.
Tumour blood flow
There was a wide variation in baseline BF, ranging from 10.5 to 125.0 ml/100 g/min but mean BF values did not differ between the two centres. Mean baseline BF was 39.2 ± 29.9 ml/100 g/min. After starting treatment, BF decreased in all but one patient after 3 weeks (this patient showed a subsequent decrease in BF at week 6) and all except one patient after 6 weeks (this patient had an initial decrease at week 3) compared with baseline. An example is shown in Fig. 1. The mean perfusion decreased significantly to 15.1 ± 16.5 ml/100 g/min (range 0 to 60.0 ml/100 g/min; p < 0.001) at week 3 with a mean decrease of 60 ± 37% (range −100 to +7%) and to 9.4 ± 15.4 ml/100 g/min (range 0 to 66.4 ml/100 g/min; p < 0.001) at week 6 with a mean decrease of 72 ± 35% (range −100 to +18%) compared with baseline (Fig. 2). As a result of extensive necrosis and/or cavitation of the primary tumour, BF was not measurable in seven patients at week 3 and nine patients at week 6, and was recorded as being zero. Baseline BF was lower in patients who developed extensive cavitations (31.2 ± 21.0 ml/100 g/min) compared with those who did not (50.0 ± 35.2 ml/100 g/min), p = 0.012.
Tumour blood flow and anatomical response evaluation
Baseline BF was not significantly different in responders versus non-responders according to both the RECIST (mean 28.3 ± 12.3 ml/min/100 g versus 34.3 ± 21.3 ml/100 g/min, p = 0.79) and the Crabb methods (mean 27.1 ± 10.8 ml/100 g/min versus 38.6 ± 24.5 ml/100 g/min, p = 0.38) (Fig. 3).
After 3 weeks of treatment RECIST responders had a significantly lower tumour BF than non-responders (mean 4.2 ± 7.8 ml/100 g/min versus 17.7 ± 13.4 ml/100 g/min, p = 0.03; Fig. 3 a). SD was the best tumour response in the patient with an increase in tumour BF at week 3. After 6 weeks all RECIST responders had necrosis and/or extensive cavitation of the primary tumour with a BF value set to zero. Three additional patients, considered to be RECIST non-responders, had necrosis and/or tumour cavitation with no measurable BF. BF in responders was also significantly lower than in non-responders at week 6 (mean 0 ± 0 versus 13.4 ± 16.9 ml/100 g/min, p = 0.04; Fig. 3a). SD was the best tumour response in the patient with an increase in BF at week 6.
When incorporating the Crabb criteria into the objective response assessment all but one out of the seven patients in whom BF was not measurable after 3 weeks and all but one out of the nine patients in whom the BF was not measurable at week 6 were considered responders. BF after 3 and 6 weeks of treatment were lower in responders than in non-responders (mean 6.5 ± 9.5 ml/100 g/min versus 20.8 ± 13.2 ml/100 g/min, p = 0.01 and mean 3.2 ± 5.9 ml/100 g/min versus 17.3 ± 19.8 ml/100 g/min, p = 0.01 respectively; Fig. 3b). Again, SD was the best tumour response in the two patients with an increase in tumour BF at weeks 3 and 6.
Regarding the percentage change in BF, RECIST responders demonstrated a significantly larger decrease in BF after 3 weeks (mean −85 ± 30% versus −49 ± 34%, p = 0.03) and 6 weeks of treatment (mean −100 ± 0% versus −60 ± 36%, p = 0.004) (Fig. 4a). This was similarly seen in Crabb responders versus non-responders at weeks 3 (−80 ± 30% versus −40 ± 32%, p = 0.01) and 6 (−88 ± 22% versus −51 ± 38 %, p = 0.01; Fig. 4b).
Bar chart showing percentage change in tumour blood flow according to a RECIST tumour response and b tumour response according to Crabb. Responders (unshaded blocks) had a significantly larger decrease in blood flow after 3 and 6 weeks of treatment compared with non-responders (shaded blocks) according to both response methods
Tumour blood flow and progression-free/overall survival
There was no association between pre-treatment absolute BF values relative to the median and PFS. Patients with a decrease larger than the median decrease of 91% at week 6 showed a trend towards a longer PFS than those with a smaller decrease (7.1 months [95% CI 5.1–9.0] versus 5.7 [95% CI 0–14.2], p = 0.06; Fig. 5). This was not apparent at week 3. Neither absolute change nor change in BF relative to the median was associated with OS.
Discussion
This is, to our knowledge, the first study to assess tumour perfusion measured by DCE-CT in patients with NSCLC receiving anti-angiogenic and anti-EGFR therapy. We found serial DCE-CTs to be feasible, well tolerated and easily integrated into the routine clinical practice with perfusion measurements possible in most patients. Tumour cavitation, beam-hardening artefacts and small tumour size were the main reasons why perfusion could not be reliably measured. The last two of these factors are known to prohibit perfusion measurements [27, 28].
Tumour cavitation frequently occurs in patients receiving angiogenesis inhibitors [20]. We found that baseline tumour BF was lower in patients who developed extensive cavitations compared with those who did not. In some cases cavitations posed a challenge for BF measurements. On the one hand, when the cavitation was limited, BF in the remaining tumour volume could be quantified. However, perfusion is not distributed homogeneously throughout tumours [27, 29]. Measuring average BF in the remaining peripheral tumour volume may overestimate BF and thus, in part, explain the wide variation in BF seen. On the other hand, in some patients cavitation was so extensive that insufficient tumour tissue remained to enable a reliable measurement of BF and this was recorded as zero. Although, we assigned a value of zero in these patients it is clear that BF was not truly absent, but simply not measurable by DCE-CT.
Although the sample size is small and there was a wide variation in absolute BF levels, tumour BF decreased in all but one patient after 3 and 6 weeks of treatment with sorafenib and erlotinib. Additionally, the mean BF of the whole group decreased significantly following 3 and 6 weeks of treatment. This may reflect the proposed anti-angiogenic mechanism of action of sorafenib. Bevacizumab, a monoclonal antibody angiogenesis inhibitor, has similarly been shown to reduce tumour perfusion in colorectal cancer [7, 10, 30]. For NSCLC, a reduction in tumour blood volume is reported after administration of combretastatin, a vascular disrupting agent, in patients who had an increase in tumour permeability following radiotherapy and after treatment with N-nitro-l-arginine, a nitric oxide synthesis (NOS) inhibitor [29, 31].
On the other hand, other studies have also shown changes in tumour vascular parameters in patients with NSCLC receiving “non-vascular targeting” therapies. Kiessling et al. describe a reduction in tumour perfusion in a patient after two cycles of chemotherapy [27]. Wang et al. found a significant decrease in blood flow and volume in patients responding to (chemo)radiotherapy [32]. The decrease in tumour BF in our study may thus reflect a general therapy effect.
Currently standard response assessment by RECIST is based on anatomical measurements with response defined as a decrease in tumour size greater than 30%. However, anatomical changes lag behind functional changes and responses based on anatomical assessments may become apparent after prolonged treatment duration. We demonstrated a significant difference in tumour perfusion in objective (anatomical) responders compared with non-responders after only 3 weeks of treatment, with responders having lower BF levels than non-responders. This is partly explained by the development of extensive cavitations in responders as discussed above. More importantly, however, a larger decline in BF after 6 weeks of treatment was associated with a trend towards a longer PFS. To our knowledge, only one other study has investigated the correlation between the anti-vascular effect of treatment and outcome in NSCLC. Although there were no significant changes in the whole group, responders to (chemo)radiotherapy had a significant decrease in tumour blood flow and volume, and patients with a decrease in permeability–surface area product had a longer PFS and OS [32].
Despite the encouraging results of our study, a number of unresolved issues remain regarding DCE-CT. The optimal timing of response assessment of anti-angiogenic therapy is unknown. Jain’s concept of a transient normalisation of tumour vasculature and improved tumour BF with anti-angiogenic treatment, followed by vascular pruning and a reduction in tumour blood flow, is estimated to occur within the first week of anti-angiogenic therapy [7, 33, 34]. We performed our first follow-up DCE-CT after 3 weeks of treatment and therefore most likely beyond this normalisation period. Another question concerns the reproducibility of whole tumour perfusion measurements [11]. We plan to determine the reproducibility of our perfusion measurements in a follow-up study. Additionally, the magnitude of change in tumour perfusion which is clinically relevant and the optimal temporal resolution of DCE-CT for the calculation of tumour vascular parameters remain unknown. In stroke and colorectal patients acquisition intervals of more than 1 s and more than 3 s, respectively, influence perfusion measurements [35–37]. We used a temporal resolution of 4 s because a higher temporal resolution would not have permitted whole tumour measurements.
Limitations of DCE-CT are the current lack of standardised protocols [38], the requirement of breath holding during image acquisition to prevent motion artefact and beam-hardening artefacts of tumours located close to large central blood vessels giving potentially false positive results. However, compared with other imaging techniques used to assess tumour perfusion, DCE-CT is cheap, simple, widely available, has high spatial resolution and can be integrated into existing CT protocols.
In conclusion, serial DCE-CTs appear to be feasible in patients with NSCLC. Using this technique we demonstrated a decrease in tumour blood flow following sorafenib and erlotinib therapy. Moreover, early changes in blood flow were predictive of objective response and tended to indicate a longer progression-free survival. Further studies are needed but our data suggest that tumour perfusion may be valuable in the early response monitoring of anti-angiogenic agents, with larger reductions reflecting greater treatment efficacy. Early response methods will enable earlier discontinuation of ineffective treatment, thereby limiting unnecessary side effects and enabling earlier switching to other, potentially effective, therapy.
References
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Therasse P (2002) Measuring the clinical response. What does it mean? Eur J Cancer 38:1817–1823
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Fox SB, Harris AL (2004) Histological quantitation of tumour angiogenesis. APMIS 112:413–430
Meijerink MR, van Cruijsen H, Hoekman K, Kater M, van Schaik C, van Waesberghe JH, Giaccone G, Manoliu RA (2007) The use of perfusion CT for the evaluation of therapy combining AZD2171 with gefitinib in cancer patients. Eur Radiol 17:1700–1713
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145–147
Xiong HQ, Herbst R, Faria SC, Scholz C, Davis D, Jackson EF, Madden T, McConkey D, Hicks M, Hess K, Charnsangavej CA, Abbruzzese JL (2004) A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs 22:459–466
Thomas JP, Arzoomanian RZ, Alberti D, Marnocha R, Lee F, Friedl A, Tutsch K, Dresen A, Geiger P, Pluda J, Fogler W, Schiller JH, Wilding G (2003) Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol 21:223–231
Koukourakis MI, Mavanis I, Kouklakis G, Pitiakoudis M, Minopoulos G, Manolas C, Simopoulos C (2007) Early antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imaging. Am J Clin Oncol 30:315–318
Ng QS, Goh V, Fichte H, Klotz E, Fernie P, Saunders MI, Hoskin PJ, Padhani AR (2006) Lung cancer perfusion at multi-detector row CT: reproducibility of whole tumor quantitative measurements. Radiology 239:547–553
Ng QS, Goh V, Klotz E, Fichte H, Saunders MI, Hoskin PJ, Padhani AR (2006) Quantitative assessment of lung cancer perfusion using MDCT: does measurement reproducibility improve with greater tumor volume coverage? AJR Am J Roentgenol 187:1079–1084
Tateishi U, Nishihara H, Watanabe S, Morikawa T, Abe K, Miyasaka K (2001) Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic-pathologic correlation. J Comput Assist Tomogr 25:23–27
Li Y, Yang ZG, Chen TW, Chen HJ, Sun JY, Lu YR (2008) Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung Cancer 61:44–53
Lind JS, Dingemans AM, Groen HJ, Thunnissen FB, Bekers O, Heideman DA, Honeywell RJ, Giovannetti E, Peters GJ, Postmus PE, van Suylen RJ, Smit EF. A multicenter phase II study of erlotinib and sorafenib in chemotherapy-naive patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010 Jun 1;16(11):3078–87
Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O'Leary J, Reck M (2009) Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol 27:4274–4280
Liu B, Barrett T, Choyke P, Maynard K, Wright J, Kummar S, Murgo A, Doroshow J, Gutierrez M, Nci B (2006) A phase II study of BAY 43-9006 (sorafenib) in patients with relapsed non-small cell lung cancer (NSCLC). J Clin Oncol (Meeting Abstracts) 24:17119
Schiller JH, Lee JW, Hanna NH, Traynor AM, Carbone DP (2008) A randomized discontinuation phase II study of sorafenib versus placebo in patients with non-small cell lung cancer who have failed at least two prior chemotherapy regimens: E2501. J Clin Oncol (Meeting Abstracts) 26:8014
Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, Leighl NB, Shepherd FA, Powers J, Seymour L, Laurie SA (2008) Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol 27:404–410
Zankl M, Panzer W, Drexel G (1993) Tomographic anthropomorphic models II. Organ doses from tomographic examinations. GSF report 30/93. Neuherberg, Germany
Zankl M, Panzer W, Drexel G (1991) The calculation of dose from external photon exposures using reference humans and Monte Carlo methods IV. Organ doses from tomographic examinations. GSF report 30/91. Neuherberg, Germany
Stamm G, Nagel HD (2002) CT-expo—a novel program for dose evaluation in CT. Rofo 174:1570–1576
Tsushima Y, Funabasama S, Sanada S, Aoki J, Endo K (2002) Development of perfusion CT software for personal computers. Acad Radiol 9:922–926
Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K (2004) Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol 11:215–223
Zhang M, Kono M (1997) Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology 205:471–478
Kiessling F, Boese J, Corvinus C, Ederle JR, Zuna I, Schoenberg SO, Brix G, Schmahl A, Tuengerthal S, Herth F, Kauczor HU, Essig M (2004) Perfusion CT in patients with advanced bronchial carcinomas: a novel chance for characterization and treatment monitoring? Eur Radiol 14:1226–1233
Ovali GY, Sakar A, Goktan C, Celik P, Yorgancioglu A, Nese N, Pabuscu Y (2007) Thorax perfusion CT in non-small cell lung cancer. Comput Med Imaging Graph 31:686–691
Ng QS, Goh V, Carnell D, Meer K, Padhani AR, Saunders MI, Hoskin PJ (2007) Tumor antivascular effects of radiotherapy combined with combretastatin a4 phosphate in human non-small-cell lung cancer. Int J Radiat Oncol Biol 67:1375–1380
Willett CG, Boucher Y, Duda DG, di Tomaso E, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC, Sahani DV, Kalva SP, Cohen KS, Scadden DT, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Shellito PC, Mino-Kenudson M, Lauwers GY (2005) Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 23:8136–8139
Ng QS, Goh V, Milner J, Stratford MR, Folkes LK, Tozer GM, Saunders MI, Hoskin PJ (2007) Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol 8:111–118
Wang J, Wu N, Cham MD, Song Y (2009) Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol 193:1090–1096
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–563
Kloska SP, Fischer T, Sauerland C, Buerke B, Dziewas R, Fischbach R, Heindel W (2010) Increasing sampling interval in cerebral perfusion CT: limitation for the maximum slope model. Acad Radiol 17:61–66
Kamena A, Streitparth F, Grieser C, Lehmkuhl L, Jamil B, Wojtal K, Ricke J, Pech M (2007) Dynamic perfusion CT: optimizing the temporal resolution for the calculation of perfusion CT parameters in stroke patients. Eur J Radiol 64:111–118
Goh V, Liaw J, Bartram CI, Halligan S (2008) Effect of temporal interval between scan acquisitions on quantitative vascular parameters in colorectal cancer: implications for helical volumetric perfusion CT techniques. AJR Am J Roentgenol 191:W288–W292
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76:S36–S42
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Sobin L, Wittekind Ch (eds) (2002) TNM classification of malignant tumours, 6th edn. Wiley-Liss, New York, pp 99–103
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lind, J.S.W., Meijerink, M.R., Dingemans, AM.C. et al. Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment?. Eur Radiol 20, 2890–2898 (2010). https://doi.org/10.1007/s00330-010-1869-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1869-5