Abstract
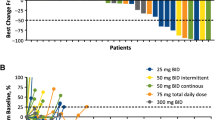
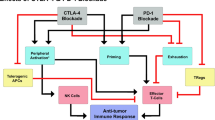
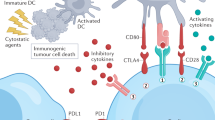
With immunotherapy enjoying a rapid resurgence based on the achievement of durable remissions in some patients with agents that derepress immune function, commonly referred to as “checkpoint inhibitors,” enormous attention developed around the IDO1 enzyme as a metabolic mediator of immune escape in cancer. In particular, outcomes of multiple phase 1/2 trials encouraged the idea that small molecule inhibitors of IDO1 may improve patient responses to anti-PD1 immune checkpoint therapy. However, recent results from ECHO-301, the first large phase 3 trial to evaluate an IDO1-selective enzyme inhibitor (epacadostat) in combination with an anti-PD1 antibody (pembrolizumab) in advanced melanoma, showed no indication that epacadostat provided an increased benefit. Here we discuss several caveats associated with this failed trial. First is the uncertainty as to whether the target was adequately inhibited. In particular, there remains a lack of direct evidence regarding the degree of IDO1 inhibition within the tumor, and previous trial data suggest that sufficient drug exposure may not have been achieved at the dose tested in ECHO-301. Second, while there is a mechanistic rationale for the combination tested, the preclinical data were not particularly compelling. More efficacious combinations have been demonstrated with DNA damaging modalities which may therefore be a more attractive alternative. Third, as a highly selective IDO1 inhibitor, epacadostat was advanced aggressively despite preclinical genetic evidence of tumors bypassing IDO1 blockade. Indeed, a well-grounded literature starting in 2011 points to targeting strategies that account for both IDO and tryptophan 2,3-dioxygenase as more appealing directions to pursue, including dual inhibitors and inhibitors of nodal downstream effector pathways such as aryl hydrocarbon receptor blockade. Overall, the clinical readout from a single trial with significant limitations is by no means a definitive test for the field. While biomarker information yet to be gleaned from ECHO-301 may yet reveal useful information regarding IDO1 pathway drugs, better rationalized compounds and better rationalized trial designs will be important in the future to accurately gauge medical impact.


Similar content being viewed by others
References
Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ (2017) Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res 77:6795–6811
Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ (2018) Indoleamine 2,3-dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol 336:175–203
Prendergast GC, Mondal A, Dey S, Laury-Kleintop LD, Muller AJ (2018) Inflammatory reprogramming with IDO1 inhibitors: turning immunologically unresponsive ‘cold’ tumors ‘hot’. Trends Cancer 4:38–58
Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O (1981) Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A 78:129–132
Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, Herve C, Gutierrez-Roelens I, Marbaix E, Sempoux C, Van den Eynde BJ (2015) Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res 3:161–172
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH (2007) Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 396:203–213
Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC (2007) Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res 67:7082–7087
Prendergast GC, Metz R, Muller AJ, Merlo LM, Mandik-Nayak L (2014) IDO2 in immunomodulation and autoimmune disease. Front Immunol 5:585
Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM (2016) Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov 6:270–285
Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC (2016) Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov 6:256–269
Munn DH, Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34:137–143
Cheong JE, Sun L (2018) Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy—challenges and opportunities. Trends Pharmacol Sci 39:307–325
Prendergast GC (2011) Cancer: why tumours eat tryptophan. Nature 478:192–194
Smith C, Chang MY, Parker KH, Beury DW, Duhadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ (2012) IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2:722–735
Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, Besmer P, Guo T, Antonescu CR, Taguchi T, Yuan J, Wolchok JD, Allison JP, Dematteo RP (2011) Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 17:1094–1100
Banerjee T, Duhadaway JB, Gaspari P, Sutanto-Ward E, Munn DH, Mellor AL, Malachowski WP, Prendergast GC, Muller AJ (2008) A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene 27:2851–2857
Muller AJ, DuHadaway JB, Jaller D, Curtis P, Metz R, Prendergast GC (2010) Immunotherapeutic suppression of indoleamine 2,3-dioxygenase and tumor growth with ethyl pyruvate. Cancer Res 70:1845–1853
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP (2013) Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210:1389–1402
Muller AJ, DuHadaway JB, Sutanto-Ward E, Donover PS, Prendergast GC (2005) Inhibition of indoleamine 2,3-dioxygenase, an immunomodulatory target of the tumor suppressor gene Bin1, potentiates cancer chemotherapy. Nat Med 11:312–319
Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, Prendergast GC, Mellor AL (2008) Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A 105:17073–17078
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van Den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274
Qiang Zhang, Yan Zhang, Jason Boer, Jack G. Shi, Peidi Hu, Sharon Diamond, Swamy Yeleswaram, (2017) In Vitro Interactions of Epacadostat and its Major Metabolites with Human Efflux and Uptake Transporters: Implications for Pharmacokinetics and Drug Interactions. Drug Metabolism and Disposition 45 (6):612-623
Beatty GL, O'Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA, Newton RC, Schaub R, Maleski J, Leopold L, Gajewski TF (2017) First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res 23:3269–3276
Yue EW, Sparks R, Polam P, Modi D, Douty B, Wayland B, Glass B, Takvorian A, Glenn J, Zhu W, Bower M, Liu X, Leffet L, Wang Q, Bowman KJ, Hansbury MJ, Wei M, Li Y, Wynn R, Burn TC, Koblish HK, Fridman JS, Emm T, Scherle PA, Metcalf B, Combs AP (2017) INCB24360 (epacadostat), a highly potent and selective indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor for immuno-oncology. ACS Med Chem Lett 8:486–491
Giannakis M, Li H, Jin C, Rodiv SG, Desai K, Horak C (2017) Metabolomic correlates of response in nivolumab-treated renal cell carcinoma and melanoma patients. Presented at the American Society of Clinical Oncology (ASCO) 2017 Annual Meeting, Chicago, IL Abstract 3036
Luke JJ, Gelmon K, Pachynski RK, Desai J, Moreno V, Tabernero JM, Gomez-Roca CA, Chu Q, Basciano P, Phillips P, Zhu L, Liu Z, Siu LL (2017) O41 preliminary antitumor and immunomodulatory activity of BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, in combination with nivolumab in patients with advanced cancers. Presented at the 32nd Annual Meeting of the Society for Immunotherapy of Cancer (SITC 2017), National Harbor, MD Abstract O41
Rose S (2017) Epacadostat shows value in two SCCHN trials. Cancer Discov 7:OF2
Siu LL, Gelmon K, Chu Q, Pachynski R, Alese O, Basciano P, Walker J, Mitra P, Zhu L, Phillips P, Hunt J, Desai J (2017) BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Presented at the 2017 Annual Meeting of the American Association for Cancer Research (AACR), Washington, DC Abstract CT116
Zakharia Y, McWilliams R, Shaheen M, Grossman K, Drabick J, Milhem M, Rixie O, Khleif S, Lott R, Kennedy E, Munn D, Vahanian N, Link C (2017) Interim analysis of the phase 2 clinical trial of the IDO pathway inhibitor indoximod in combination with pembrolizumab for patients with advanced melanoma. Presented at the 2017 Annual Meeting of the American Association for Cancer Research (AACR), Washington, DC Abstract CT117
Daud A, Saleh MN, Hu J, Bleeker JS, Riese MJ, Meier R, Zhou L, Serbest G, Lewis KD (2018) Epacadostat plus nivolumab for advanced melanoma: updated phase 2 results of the ECHO-204 study. Presented at the American Society of Clinical Oncology (ASCO) 2018 Annual Meeting, Chicago, IL Abstract 9511
Gibney GT, Hamid O, Gangadhar TC, Lutzky J, Olszanski AJ, Gajewski T, Chmielowski B, Boasberg PD, Zhao Y, Newton RC, Scherle PA, Bowman J, Maleski J, Leopold L, Weber JS (2014). Preliminary results from a phase 1/2 study of INCB024360 combined with ipilimumab (ipi) in patients (pts) with melanoma. Presented at the American Society of Clinical Oncology (ASCO) 2014 Annual Meeting, Chicago, IL Abstract 3010
Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca S-B, Bolduc AK, Hoang K, Ashley C, McCall D, Rojiani AM, Maria BL, Rixe O, MacDonald TJ, Heeger PS, Mellor AL, Munn DH, Johnson TS (2014) The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer 2:21
Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, Chen M, Kol A, Shiao SL, Reddy A, Perks JR, Culp WTN, Sparger EE, Canter RJ, Sckisel GD, Murphy WJ (2016) Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res 22:4328–4340
Johnson TS, McGaha T, Munn DH (2017) Chemo-immunotherapy: role of indoleamine 2,3-dioxygenase in defining immunogenic versus tolerogenic cell death in the tumor microenvironment. Adv Exp Med Biol 1036:91–104
Ladomersky E, Zhai L, Lenzen A, Lauing KL, Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F, Gong W, Liu Y, Jiang B, Tang T, Patel R, Platanias LC, James CD, Stupp R, Lukas RV, Binder DC, Wainwright DA (2018) IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin Cancer Res 24:2559–2573
Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL (2016) STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res 76:2076–2081
Wang W, Huang L, Jin JY, Jolly S, Zang Y, Wu H, Yan L, Pi W, Li L, Mellor AL, Kong FS (2018) IDO immune status after chemoradiation may predict survival in lung cancer patients. Cancer Res 78:809–816
Nevler A, Muller AJ, Sutanto-Ward E, DuHadaway JB, Nagatomo K, Londin E, O’Hayer K, Cozzitorto JA, Lavu H, Yeo TP, Curtis M, Villatoro T, Leiby BE, Winter JM, Yeo CJ, Prendergast GC, Brody JR (2018) Host IDO2 gene status influences tumor progression and radiotherapy response in KRAS-driven sporadic pancreatic cancers. Clin Cancer Res. provisionally accepted
Muller AJ, Mandik-Nayak L, Prendergast GC (2010) Beyond immunosuppression: reconsidering indoleamine 2,3-dioxygenase as a pathogenic element of chronic inflammation. Immunotherapy 2:293–297
Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ (2014) Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 63:721–735
Muller AJ, Scherle PA (2006) Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer 6:613–625
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203
D'Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC, Richer JK (2015) A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res 75:4651–4664
Mondal A, Smith C, DuHadaway JB, Sutanto-Ward E, Prendergast GC, Bravo-Nuevo A, Muller AJ (2016) IDO1 is an integral mediator of inflammatory neovascularization. EBioMedicine 14:74–82
Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, Terness P (2009) IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58:153–157
Witkiewicz A, Williams TK, Cozzitorto J, Durkan B, Showalter SL, Yeo CJ, Brody JR (2008) Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 206:849–854 discussion 54-6
Witkiewicz AK, Costantino CL, Metz R, Muller AJ, Prendergast GC, Yeo CJ, Brody JR (2009) Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg 208:781–787 discussion 7-9
Nevler A, Muller AJ, Cozzitorto JA, Goetz A, Winter JM, Yeo TP, Lavu H, Yeo CJ, Prendergast GC, Brody JR (2018) A sub-type of familial pancreatic cancer: evidence and implications of loss-of-function polymorphisms in indoleamine-2,3-dioxygenase-2. J Am Coll Surg 226:596–603
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL (2005) GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22:633–642
Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H (2009) Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A 106:12055–12060
Eleftheriadis T, Pissas G, Yiannaki E, Markala D, Arampatzis S, Antoniadi G, Liakopoulos V, Stefanidis I (2013) Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte reaction affects glucose influx and enzymes involved in aerobic glycolysis and glutaminolysis in alloreactive T-cells. Hum Immunol 74:1501–1509
Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC (2012) IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 1:1460–1468
Prendergast GC, Fox E, Oliver T, Rowe M, Thomas S, Zakharia Y, Gilman PB, Muller AJ (2018) Indoximod: an immunometabolic adjuvant that empowers T cell activity in cancer. Front Oncol. in press
Joshi M, Kulkarni A, Pal JK (2013) Small molecule modulators of eukaryotic initiation factor 2alpha kinases, the key regulators of protein synthesis. Biochimie 95:1980–1990
Zhang YJ, Duan Y, Zheng XF (2011) Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today 16:325–331
Gutierrez-Vazquez C, Quintana FJ (2018) Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48:19–33
Safe S, Lee SO, Jin UH (2013) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci 135:1–16
Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, Qerqez A, Dekker JD, Tanno Y, Lu WC, Karamitros CS, Ford K, Tan B, Zhang XM, McGovern K, Coma S, Kumada Y, Yamany MS, Sentandreu E, Fromm G, Tiziani S, Schreiber TH, Manfredi M, Ehrlich LIR, Stone E, Georgiou G (2018) Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol 36: 758–764
Acknowledgements
G.C.P. and A.J.M. acknowledge grant support from the NCI (R01 CA191119) and the W.W. Smith Trust, with additional support from the Lankenau Medical Center Foundation and the Main Line Health System. G.C.P. holds The Havens Chair for Biomedical Research at the Lankenau Institute for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G.C.P. and A.J.M. are shareholders and G.C.P. is a former compensated scientific advisor and grant recipient for New Link Genetics Corporation, a biopharmaceutical company that has licensed IDO intellectual property for clinical development from the Lankenau Institute of Medical Research, as described in U.S. Patents Nos. 7705022, 7714139, 8008281, 8058416, 8383613, 8389568, 8436151, 8476454, and 8586636. G.C.P. is a compensated scientific advisor for Kyn Therapeutics Inc. which is developing IDO/TDO/AHR pathway antagonists for cancer treatment. A.J.M. is a grant recipient and compensated scientific advisor for I-O Biotech AG which is developing IDO vaccines for cancer treatment. MM is a shareholder in Kyn Therapeutics. Y.Z. is a recipient of research and conference travel support from NewLink Genetics and an advisory board member for Amgen, Roche Diagnostics, Novartis, Eisai, Castle Bioscience, and Exelixis.
Additional information
This article is a contribution to the special issue on Anti-cancer immunotherapy: Breakthroughs and Future strategies - Guest Editor: Mads Hald Andersen
Rights and permissions
About this article
Cite this article
Muller, A.J., Manfredi, M.G., Zakharia, Y. et al. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol 41, 41–48 (2019). https://doi.org/10.1007/s00281-018-0702-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-018-0702-0