Abstract
Purpose
The drug label of sunitinib includes a warning for concomitant use of grapefruit juice (GJ) but clinical evidence for this drug interaction is lacking. The aim of this study is to determine the effect of GJ, a potent intestinal cytochrome P450 (CYP) 3A4 inhibitor, on steady-state sunitinib pharmacokinetics (PK).
Methods
Sunitinib PK was evaluated in eight cancer patients receiving sunitinib monotherapy in a “4 weeks on—2 weeks off” dose regimen. Serial blood samples for PK analysis of sunitinib were collected on two separate days. On both PK days, patients received a single oral dose of 7.5-mg midazolam as a phenotypic probe for assessment of intestinal CYP3A4 activity. The first PK day was at steady-state sunitinib PK (between days 14–20), the second PK day was on day 28. On days 25, 26 and 27, 200-mL GJ was consumed 3 times a day. The effect of GJ on sunitinib exposure was assessed by comparing sunitinib PK with and without GJ.
Results
Concomitant use of GJ and sunitinib resulted in an 11% increase of the relative bioavailability of sunitinib (P < 0.05). The effect of GJ on CYP3A4 activity was confirmed by an increase of ~50% of mean midazolam exposure (AUC0–24 h) from 122.1 to 182.0 ng h/mL (P = 0.034).
Conclusion
GJ consumption results in a marginal increase in sunitinib exposure which is not considered clinically relevant. There is no clinical evidence underscoring the warning in the sunitinib drug label regarding concomitant use of GJ.
Similar content being viewed by others
Introduction
Sunitinib malate (Sutent®; SU11248) is a multitarget tyrosine kinase inhibitor approved for the first-line treatment of metastatic renal cell carcinoma (mRCC) and imatinib-resistant metastatic gastrointestinal stromal tumors (GIST) [1–3]. The licensed dosing regimen for sunitinib is a “4 weeks on—2 weeks off” schedule [4]. Sunitinib is absorbed from the gastrointestinal tract to an unknown extent. The intake of food does not affect the pharmacokinetics of sunitinib [5]. Sunitinib is in vitro extensively protein bound, has a long half-life of ~50 h and a high apparent volume of distribution of ~2,000 liters [3, 6]. CYP3A4 metabolizes sunitinib into an active metabolite, SU12662, which is further metabolized by CYP3A4 into inactive moieties [3, 7, 8]. Sunitinib has not been described to be a substrate of any other metabolizing enzymes besides CYP3A4. It was identified in vitro as a moderate substrate of the ATP-binding cassette (ABC) drug transporters ABCG2 and ABCB1 and showed no affinity for organic anion transporting polypeptides (OATPs). However, the clinical relevance of these transporters needs to be investigated in vivo [9, 10].
Coadministration of ketoconazol, a potent CYP3A4 inhibitor, results in a 51% increase of the combined area under the concentration time curve (AUC) of sunitinib and SU12662 after a single dose of sunitinib in healthy volunteers [3]. This observation was extrapolated to warnings for the potential effect of strong CYP3A4 inhibitors including grapefruit juice (GJ) in the drug label of sunitinib [8].
GJ contains a rich mixture of several hundred ingredients which may be responsible for the GJ—drug interaction effect [11–14]. However, by administrating the purified forms of the different compounds to human volunteers, the furanocoumarins (most abundant bergamottin (BG) and 6′,7′-dihydroxybergamottin (DHB)) were confirmed to result in a significant CYP3A4 inhibiting effect, and therefore these constituents are held responsible for GJ—drug interactions [15–17]. GJ is an inhibitor of intestinal CYP3A4, with little effect on hepatic CYP3A4 activity [18]. Grapefruit juice also appears to be an inhibitor of ABCB1 and possibly of OATP located in the intestines [17–20].
Recently, multiple oral anticancer therapies, mainly tyrosine kinase inhibitors, are introduced, and since most of them are substrates of CYP3A4, their drug label mentions that grapefruit juice could increase their exposure. Notably, only two studies have specifically determined the clinical effect of GJ on an oral anticancer drugs (etoposide and nilotinib), and inconsequent effects were observed, namely a decrease in etoposide exposure but an increase in nilotinib exposure [21, 22]. Therefore, the evidence for GJ—drug interaction of most anticancer drugs is lacking. Since patients are increasingly being treated with oral anticancer therapy in the recent years, it is highly relevant to better understand and to determine potential GJ—drug interactions of oral anticancer drugs. Therefore, in this study the effect of concomitant use of GJ on sunitinib exposure in patients with cancer was determined.
Materials and methods
Patients
Patients eligible for study entry were treated with sunitinib at a dose level of 25–50 mg once daily in a “4 weeks on—2 weeks off” regimen. The patient should have a tumor for which sunitinib is registered as the first-line (metastatic renal cell carcinoma), second-line (gastrointestinal stromal tumor) treatment or for which no treatment options were available anymore. All patients were ≥18 years old and had a WHO performance status ≤2 and a life expectancy of at least 12 weeks. Cytotoxic chemotherapy or radiation therapy within 4 weeks prior to entering the study protocol was not allowed. Concurrent use of substances known or likely to interfere with the pharmacokinetics (PK) of sunitinib and with CYP3A4 activity, such as ketoconazol, fluconazol, rifampicin and St. John’s wort, was not allowed within 14 days before study entry and during the study. All patients had adequate bone marrow, renal and hepatic functions as defined by hemoglobin ≥6.0 mmol/L, WBC ≥3.0 × 109/L, platelets ≥100 × 109/L, creatinine clearance ≥60 mL/min, bilirubin ≤1.75 × the upper limit of institutional normal range. Prior to commencing the study, a sample size of eight patients was determined as sufficient for a paired, two-sided analysis to detect a difference of 25% in sunitinib exposure with a power (1-β) of 0.8, and a two-sided significance level (α) of 0.05. The study was approved by the institutional ethics committee (Leiden University Medical Center, The Netherlands), and all patients gave written informed consent before entering the study.
Study design
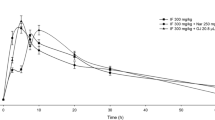
The study was designed to evaluate the effect of GJ on steady-state sunitinib PK. All patients were treated with commercially available hard capsule sunitinib malate (Pfizer, Kent, United Kingdom) at an oral dose of 25/37.5/50 mg once daily in a “4 weeks on followed by 2 weeks off” dose regimen. The study was performed during one sunitinib treatment cycle of 6 weeks. Patients were admitted to the hospital on two separate PK days. The first PK day was at steady-state sunitinib PK (between day 14–20) and the second PK day was on day 28. On days 25, 26 and 27, the patients used 200-ml grapefruit juice of a preselected lot of commercially available GJ 3 times daily. On these 3 days, sunitinib was simultaneously used with the morning consumption of the grapefruit juice. On both PK days, patients were given one midazolam 7.5-mg tablet (Roche, Woerden, The Netherlands) as a phenotypic probe to confirm the inhibitory effect of GJ on intestinal CYP3A4 activity (Fig. 1).
Selection of a grapefruit juice batch
Different batches of GJ show a considerable variability in BG (~35 fold) and DHB (~200 fold) concentration [23]. Therefore, selecting a batch with a sufficient amount of BG and DHB to induce a clinically relevant effect on CYP3A4 substrates was necessary before the interaction study was conducted [15].
Concentrations of BG and DHB were quantified in various batches of GJ from different brands using a validated high-performance liquid chromatography—ultraviolet detection (HPLC–UV) method. This assay was based on a previously published method with minor modifications.[23]. Briefly, the juice was homogenized by shaking. GJ (0.5 mL) was mixed with 10-μL internal standard (100 μg/mL, in methanol) and 2-mL ethyl acetate. Calibration standards containing 0.2–4 μg/mL BG and 0.1–2 μg/mL DHB were prepared at the start of each analytical run. The standard stock solution contained BG and DHB (100 and 50 μg/mL in DMSO:methanol(1:3)). The residue from the organic phase was reconstituted with 100 μL of DMSO/acetonitril solution (1:3 v/v) and applied to the HPLC separation system (Unexas 2104, Knauer, Berlin, Germany). The compounds of interest were separated on a Hypersil ODS RP analytical column (4.6 × 100 mm, i.d 3 μm) using the following gradient [time scale (minutes–minutes)/percentage of solvent A (water 2500/phosphoric acid 1.25)/percentage of solvent B (acetonitril)]: 0-7/70/30, 7-17 70/30 → 0/100, 17–18/0/100, 18–19 0/100 → 70/30, 19–22/70/30. DHB, fenprocoumon and BG eluted at 10.9, 12.8 and 16.5 min, respectively. Linearity was confirmed over the range of 0.2–24 μg/mL for BG and 0.1–12 μg/mL for DHB. The within-day and between-day precision and accuracy were <15%.
Pharmacokinetic sampling
Blood samples were collected on the first and second PK day of the study for assessing sunitinib and midazolam plasma concentrations. Blood was collected in heparin-containing tubes at the following time points: pre-dose, 10, 20, 40 min; 1, 2, 3, 4, 5, 6, 7, 8, 10, 12 and 24 h after simultaneous intake of sunitinib and midazolam. Blood samples were centrifuged at 3,000 rpm for 10 min, the plasma was stored in two separate tubes for the midazolam and sunitinib analysis, and both tubes were stored at−80˚C until the day of analysis.
Bioanalysis of sunitinib and midazolam
Sunitinib was measured using a validated liquid chromatographic-tandem mass spectrometric (LC–MS/MS) assay, which has been described earlier [24]. The calibration curve of sunitinib was linear over the range of 0.2–500 ng/mL. The within-day and between-day precision and accuracy were <8%. The LLQ of the sunitinib assay was 0.2 ng/mL.
Midazolam was measured using a validated liquid chromatographic-tandem mass spectrometric (LC–MS/MS) assay. Briefly, 200-μl plasma was extracted by adding 500 μl of acetonitril containing midazolam D4 (4 μg/L) as the internal standard, followed by vortex mixing and centrifugation at 13,000 rpm for 5 min at ambient temperature. The supernatant was collected and 10-μL was separated on an Atlantis T3 C18 analytical column (2.1 × 50 mm, i.d 3 μm) and eluted with the following gradient [flow rate (ml/min)/time (minutes)/percentage of solvent A (formic acid 0.1% in water)/percentage of solvent B (formic acid 0.1% in acetonitril)]: 0.3/0.5/85/15/, 0.3/1/10/90, 0.3/4.3/10/90, 0.5/0.01/10/90, 0.5/0.39/85/15, 0.5/3.3/85/15, 0.3/0.05/85/15, 0.3/0.05/85/15. The effluent was monitored with a Micromass Quattro LC triple-quadruple mass-spectrometric detector (Waters, Milford, MA, USA) using the electrospray positive ionization mode. The calibration curve of midazolam was linear over the range of 1–100 ng/mL. The within-day and between-day precision and accuracy were <5%. The LLQ of the midazolam assay was 0.3 ng/mL.
Pharmacokinetic analysis of midazolam
Midazolam plasma concentrations were analyzed by non-compartmental methods using WinNonlin (version 5.2.1) (Pharsight Corporation, Mountain View, CA, USA). The midazolam area under the concentration time curve (AUC0–24 h) was calculated and was compared for each patient between the first and second PK day. Statistical analysis included the two-sided paired Student’s t test, and P values < .05 were considered statistically significant. The statistical calculations were performed using SPSS 16.0 (SPSS Inc. headquarters, Chicago, IL, USA)
Pharmacokinetic analysis of sunitinib
Sunitinib plasma concentrations were evaluated by a population pharmacokinetic method using NONMEM (version VI, level 1.0) (Globomax, Hanover, MD, USA). The first-order conditional estimation (FOCE) method of NONMEM with interaction (INTER) between the interindividual and residual random effects was used [25]. Discrimination between hierarchical models was based on comparison of the objective function values (OFV) of NONMEM using the likelihood ratio test. A decrease in ∆OFV of 6.63 was considered statistically significant (P < .01) [26].
A base model was developed to describe sunitinib PK, using sunitinib concentrations obtained on the first and second PK day. Next, a final model was developed by the introduction of a GJ effect on the relative bioavailability of sunitinib, resulting in an effect on the apparent clearance and apparent volume of distribution and thereby exposure to sunitinib, since it was assumed that GJ exerts its effect only by irreversible inhibition of intestinal CYP3A4 and possibly by inhibition of ABCB1 (Fig. 2). The recovery half-life of CYP3A4 activity after GJ consumption was set to 23 h [27].
The model was evaluated by goodness-of-fit plots, case deletion diagnostics and a numerical predictive check. Moreover, a log-likelihood profile was generated for the effect size of GJ to determine the confidence interval.
The effect of GJ on sunitinib bioavailability was studied for simultaneous intake of sunitinib and grapefruit juice but was also evaluated for sunitinib intake 7, 24, 72 h and 1 week after the last GJ consumption.
Results
Patients
Eight patients were enrolled into the study and all were evaluable for PK analysis. Patient characteristics are summarized in Table 1. No severe or unexpected side effects were observed during the 3 days of GJ or midazolam coadministration on both PK days.
Selection of grapefruit juice
The concentration of BG and DHB was measured in 6 different lots of GJ. BG and DHB concentrations among the lots tested ranged from 2.6 to 11.9 mg/L and 0.4 to 8.1 mg/L, respectively. The concentration of BG and DHB in the selected lot of GJ was 33.1 and 2.7 μmol/L, respectively, corresponding to 2.2 mg/200 mL BG and 0.2 mg/200 mL DHB. Due to the expiry date, a second lot of the same brand was selected for the last two patients of the study. The concentrations in the second lot selected were 23.5 μmol/L BG and 5.7 μmol/L DHB, corresponding to 1.6 mg/200 mL BG and 0.4 mg/200 mL DHB. The concentration of BG in both lots was sufficient to induce a significant drug interaction [15].
Pharmacokinetic analysis of midazolam
Midazolam exposure (AUC0–24 h) increased after prior intake of GJ. The midazolam exposure expressed as AUC0–24 h (±standard error of the mean (SEM)) with and without GJ was 122.1 (± 32.9) and 182.0 (± 52.2) ng h/mL, respectively (P = .034). Therefore, midazolam exposure increased with ~50% by GJ, and this observation confirms the inhibitory effect of GJ on CYP3A4 activity (Fig. 3)
Pharmacokinetic analysis of sunitinib
A one-compartment model with linear elimination and first-order absorption adequately described the concentration–time profile of sunitinib. The data did not contain sufficient information to support a two-compartment model and this was demonstrated by a non-significant improvement (ΔOFV = −0.048) of the model on introduction of a second compartment [6]. Inclusion of an absorption lag time significantly improved the base model of sunitinib. Between-subject variabilities of the absorption rate and clearance were large (60–70%). The base model of sunitinib is graphically presented in Fig. 2 (left side).
In the final model, CYP3A4 activity was depleted by each GJ consumption (9 in total), and the activity was restored with a half-life of 23 h (Fig. 4a) [27]. Inhibition of CYP3A4 activity resulted in an increase in the relative bioavailability of sunitinib (Fig. 4b). The individual predicted and measured sunitinib concentrations are depicted for all patients (Fig. 4c). Additionally, the predicted value and the individual measured concentration are depicted specifically for the both sampling days (Fig. 5). Introduction of the GJ effect on the relative bioavailability of sunitinib significantly improved the model (∆OFV = −10.01, P < .01) and resulted in the final model (Fig. 2).
The estimated PK parameters in the final model are listed in Table 2. The derived parameters are calculated with the estimated PK parameters and represent the data when GJ and sunitinib are used simultaneously. Goodness-of-fit plots demonstrated that the final model adequately described the time profile of sunitinib concentrations. Case deletion diagnostics demonstrated that the estimated GJ effect was not highly dependent on the data from a single patient (range in relative F = 1.05–1.14). Moreover, suitability of the final model was confirmed by the results from a numerical predictive check [28]. Out of 268 observed sunitinib plasma concentrations, 21.6% was below the P25–P75 (interquartile) prediction interval, 57.1% was within the interval, and 21.3% was above the P25–P75 prediction interval.
Based on the final model, it is determined that simultaneous intake of sunitinib and grapefruit juice results in a decrease of intestinal CYP3A4 activity and consequently an increase of sunitinib exposure of 11% (as a result of the increased relative bioavailability 1.11, 95%CI 1.042–1.182).
Since the intestinal CYP3A4 activity is restored with a half-life of 23 h, the relative bioavailability of sunitinib is also restored with a half-life of 23 h. The different time interval evaluations resulted in the following estimates: when GJ is consumed 7 h before the sunitinib dose, the sunitinib exposure is increased by ~8.9%, after 24 h the effect is ~5.3%, and after 72 h it is ~1.3%. If sunitinib therapy starts 1 week after the last GJ consumption, the effect of GJ on the exposure to sunitinib is negligible (~0.07%).
Discussion
This study shows that inhibition of the intestinal CYP3A4 activity by GJ results in a significant but not clinically relevant increase of sunitinib exposure. The drug label of sunitinib mentions that GJ may increase the exposure to sunitinib This remark, however, is based on extrapolation of the effect of ketoconazol on sunitinib exposure after single-dose administration, and clinical evidence for the presumably drug interaction between sunitinib and GJ is lacking [3, 8]. Our study is the first to directly investigate the effect of GJ on sunitinib exposure in patients with cancer and shows that there is the increase in sunitinib exposure by GJ consumption is clinically not relevant.
Moreover, this is the third study investigating an interaction of GJ with oral anticancer therapy. The studies show a reverse, relevant and irrelevant effect of GJ [21, 22]. Indeed, all eight approved tyrosine kinase inhibitors are substrates of CYP3A4, and therefore their exposure could be increased by the consumption of GJ. Since the tyrosine kinase inhibitors have very different bioavailabilities, the effect of GJ observed in our study cannot be extrapolated to the other tyrosine kinase inhibitors so additional studies determining the effect of GJ on the individual TKI exposure should therefore be conducted.
GJ is a potent inhibitor of intestinal CYP3A4 with little, if any, effect on the activity of hepatic CYP3A4. Indeed, a significant effect of GJ on the exposure to CYP3A4 substrates (e.g. simvastatine, felodipine and triazolam) is seen after oral administration, while the effect is only limited after intravenous administration of these drugs [15, 29–31]. GJ is also an inhibitor of the drug transporters ABCB1, OATP1A2 and OATP2B1, which could contribute to the effect of GJ on the exposure of co-administered drugs [13, 32–37].
Midazolam is extensively metabolized by CYP3A4 with less affinity for CYP3A5, ABCB1, ABCG2 and OATPs [38–41]. In previous studies, GJ showed a pronounced effect on the exposure of orally administered midazolam [27, 31, 42]. In our study, midazolam was co-administrated on both PK days as a phenotypic probe to confirm the decreased activity of intestinal CYP3A4 by the selected batch of GJ.
The patients in our study consumed GJ 3 times a day for 3 days (25, 26 and 27) at steady-state sunitinib PK. On the last sunitinib treatment day (day 28) in the 6-week treatment cycle, the sunitinib PK was determined and compared to the data obtained without consumption of GJ. The effect of GJ was estimated on the relative bioavailability of sunitinib, since GJ is a potent intestinal CYP3A4 inhibitor, and therefore, only an effect on the sunitinib uptake is expected rather than on sunitinib clearance, volume of distribution, absorption rate constant and absorption lag time. “In our study, we have focused on the effect of GJ, an intestinal CYP3A4 inhibitor, on the pharmacokinetics of sunitinib. A limited, if any, effect was expected on the SU12662 exposure since hepatic CYP3A4 activity is not interfered by GJ consumption and therefore only sunitinib was quantified”.
Indeed, the concomitant use of grapefruit juice results in a significant increase of 11% in sunitinib exposure. However, since the reported interpatient variability in sunitinib clearance is large ~40%, the effect of GJ on sunitinib exposure is negligible and should not be regarded as clinically relevant [6]. Moreover, the marginal 11% increase in sunitinib exposure is unlikely to result in an increased toxicity or different anticancer efficacy, although data on the pharmacokinetic–pharmacodynamic relationship are not available yet.
GJ irreversibly inhibits CYP3A4 and therefore it takes time to restore CYP3A4 functionality since it is dependent on formation of new enzymes. The recovery half-life of CYP3A4 activity after consuming GJ was set at 23 h according to the study of Greenblatt et al [14]. The recovery half-life was confirmed by several interaction studies between midazolam and GJ over different time intervals [31, 42, 43].
The half-life of sunitinib is relatively long (~50 h) and therefore steady-state PK of sunitinib is reached after ~8 days. Similarly, it takes ~8 days to achieve steady state after coadministration of GJ. Therefore, at the second PK day, after 3 days coadministration of GJ, steady-state sunitinib was not reached. Since a large increase of sunitinib exposure was expected upon coadministration of GJ and consequently more toxicity, it was considered unethical to continue coadministration until sunitinib steady state was reached. Therefore, we chose to analyze the effect of GJ on sunitinib PK by a compartmental PK approach. The estimated apparent clearance and volume of distribution found in our study are similar to those described earlier by compartmental approach [6]. Conversely, a non-compartmental approach was used for determining midazolam exposure used as a phenotypic probe. Indeed, we could adequately determine the exposure to midazolam with and without GJ since extensive sampling was done from the start of midazolam administration until plasma levels of midazolam were undetectable.
The lack of a clinically relevant effect of GJ on sunitinib exposure was not related to the batch of GJ that was used in this study. First, the GJ selected had a sufficient content of BG (2.2 mg/1.6 mg) to induce a significant effect on CYP3A4 activity [15]. Secondly, even after the recovery of a proportion of the intestinal CYP3A4 enzymes on the second PK day, a significant effect ~50% was observed on the phenotypic drug midazolam, which is comparable to the effect of GJ on midazolam exposure explored in earlier interaction studies [27, 42]. No effect of sunitinib on midazolam exposure was observed since midazolam exposure was similar to the exposures reported in earlier studies [42, 44]. The increase in midazolam exposure due to GJ coadministration confirms the significant effect of GJ on intestinal CYP3A4 activity. Hence, the marginal effect observed on sunitinib bioavailability is likely to be the result of the limited efficiency of sunitinib metabolism by intestinal CYP3A4. The limited effect of GJ is in contrast to the large effect (51% increase) observed after coadministration of the CYP3A4 inhibitor ketoconazol [3]. Notably, the interaction study with ketoconazol was performed after a single sunitinib dose, while in the current study the interaction with GJ was determined after multiple dosing. Inhibition of a major metabolic pathway may lead to shunting of the metabolism to alternative metabolic pathways at steady-state PK that remains unnoticed after single-dose administration. Indeed, we have reported that alternative metabolic pathways can play a dominant role after prolonged exposure to imatinib [45]. Another explanation could be that ketoconazol is a strong intestinal and hepatic CYP3A4 inhibitor, while GJ is only capable of inhibiting intestinal CYP3A4.
In conclusion, upon coadministration of GJ only marginally increases in sunitinib exposure were observed which is not regarded clinically relevant.
References
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338
Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R (2007) Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 13:1367–1373
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Bello CL, Sherman L, Zhou J, Verkh L, Smeraglia J, Mount J, Klamerus KJ (2006) Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drug 17:353–358
Houk BE, Bello CL, Kang D, Amantea M (2009) A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 15:2497–2506
Adams VR, Leggas M (2007) Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther 29:1338–1353
Pfizer (2006) Drug label Sutent approved 01/26/2006. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed on 02/18/2009. 2006
Shukla S, Robey RW, Bates SE, Ambudkar SV (2009) Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos 37:359–365
Hu S, Chen Z, Franke R, Orwick S, Zhao M, Rudek MA, Sparreboom A, and Baker SD (2009) Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin.Cancer Res (In press)
Bailey DG, Malcolm J, Arnold O, Spence JD (1998) Grapefruit juice-drug interactions. Br J Clin Pharmacol 46:101–110
Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, He K, Lown KS, Woster PM, Rahman A, Thummel KE, Fisher JM, Hollenberg PF, Watkins PB (1997) Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos 25:1228–1233
Wang EJ, Casciano CN, Clement RP, Johnson WW (2001) Inhibition of P-glycoprotein transport function by grapefruit juice psoralen. Pharm Res 18:432–438
Paine MF, Criss AB, Watkins PB (2004) Two major grapefruit juice components differ in intestinal CYP3A4 inhibition kinetic and binding properties. Drug Metab Dispos 32:1146–1153
Goosen TC, Cillie D, Bailey DG, Yu C, He K, Hollenberg PF, Woster PM, Cohen L, Williams JA, Rheeders M, Dijkstra HP (2004) Bergamottin contribution to the grapefruit juice-felodipine interaction and disposition in humans. Clin Pharmacol Ther 76:607–617
Rashid J, McKinstry C, Renwick AG, Dirnhuber M, Waller DG, George CF (1993) Quercetin, an in vitro inhibitor of CYP3A, does not contribute to the interaction between nifedipine and grapefruit juice. Br J Clin Pharmacol 36:460–463
Paine MF, Widmer WW, Hart HL, Pusek SN, Beavers KL, Criss AB, Brown SS, Thomas BF, Watkins PB (2006) A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of the grapefruit juice-felodipine interaction. Am J Clin Nutr 83:1097–1105
Saito M, Hirata-Koizumi M, Matsumoto M, Urano T, Hasegawa R (2005) Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf 28:677–694
Paine MF, Widmer WW, Pusek SN, Beavers KL, Criss AB, Snyder J, Watkins PB (2008) Further characterization of a furanocoumarin-free grapefruit juice on drug disposition: studies with cyclosporine. Am J Clin Nutr 87:863–871
Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V (2006) Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol 46:1390–1416
Reif S, Nicolson MC, Bisset D, Reid M, Kloft C, Jaehde U, McLeod HL (2002) Effect of grapefruit juice intake on etoposide bioavailability. Eur J Clin Pharmacol 58:491–494
Yin OQ, Gallagher N, Li A, Zhou W, Harrell R, Schran H (2010) Effect of grapefruit juice on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol 50:188–194
De Castro WV, Mertens-Talcott S, Rubner A, Butterweck V, Derendorf H (2006) Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem 54:249–255
Minkin P, Zhao M, Chen Z, Ouwerkerk J, Gelderblom H, Baker SD (2008) Quantification of sunitinib in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 874:84–88
Beal SL, Boeckman AJ, Sheiner LB (1988) NONMEM user’s guides. University of California at San Francisco, San Francisco CA
Wahlby U, Jonsson EN, Karlsson MO (2001) Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28:231–252
Greenblatt DJ, von Moltke LL, Harmatz JS, Chen G, Weemhoff JL, Jen C, Kelley CJ, LeDuc BW, Zinny MA (2003) Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin Pharmacol Ther 74:121–129
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20
Lilja JJ, Kivisto KT, Neuvonen PJ (2000) Duration of effect of grapefruit juice on the pharmacokinetics of the CYP3A4 substrate simvastatin. Clin Pharmacol Ther 68:384–390
Culm-Merdek KE, von Moltke LL, Gan L, Horan KA, Reynolds R, Harmatz JS, Court MH, Greenblatt DJ (2006) Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther 79:243–254
Kupferschmidt HH, Ha HR, Ziegler WH, Meier PJ, Krahenbuhl S (1995) Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther 58:20–28
Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y (2005) Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 33:518–523
Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB (2002) Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther 71:11–20
Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB (2007) Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81:362–370
Hermann M, Asberg A, Reubsaet JL, Sather S, Berg KJ, Christensen H (2002) Intake of grapefruit juice alters the metabolic pattern of cyclosporin A in renal transplant recipients. Int J Clin Pharmacol Ther 40:451–456
Bailey DG, Dresser GK, Leake BF, Kim RB (2007) Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther 81:495–502
Eagling VA, Profit L, Back DJ (1999) Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br J Clin Pharmacol 48:543–552
Franke RM, Baker SD, Mathijssen RH, Schuetz EG, Sparreboom A (2008) Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin Pharmacol Ther 84:704–709
Fuhr U, Jetter A, Kirchheiner J (2007) Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin Pharmacol Ther 81:270–283
Kim RB, Wandel C, Leake B, Cvetkovic M, Fromm MF, Dempsey PJ, Roden MM, Belas F, Chaudhary AK, Roden DM, Wood AJ, Wilkinson GR (1999) Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm Res 16:408–414
Kurnik D, Wood AJ, Wilkinson GR (2006) The erythromycin breath test reflects P-glycoprotein function independently of cytochrome P450 3A activity. Clin Pharmacol Ther 80:228–234
Farkas D, Oleson LE, Zhao Y, Harmatz JS, Zinny MA, Court MH, Greenblatt DJ (2007) Pomegranate juice does not impair clearance of oral or intravenous midazolam, a probe for cytochrome P450–3A activity: comparison with grapefruit juice. J Clin Pharmacol 47:286–294
Veronese ML, Gillen LP, Burke JP, Dorval EP, Hauck WW, Pequignot E, Waldman SA, Greenberg HE (2003) Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol 43:831–839
Mueller SC, Majcher-Peszynska J, Mundkowski RG, Uehleke B, Klammt S, Sievers H, Lehnfeld R, Frank B, Thurow K, Kundt G, Drewelow B (2009) No clinically relevant CYP3A induction after St. John’s wort with low hyperforin content in healthy volunteers. Eur J Clin Pharmacol 65:81–87
van Erp NP, Gelderblom H, Karlsson MO, Li J, Zhao M, Ouwerkerk J, Nortier JW, Guchelaar HJ, Baker SD, Sparreboom A (2007) Influence of CYP3A4 inhibition on the steady-state pharmacokinetics of imatinib. Clin Cancer Res 13:7394–7400
Acknowledgments
We thank Alex Sparreboom for the discussion on trial design.
Conflict of interest statement
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Erp, N.P., Baker, S.D., Zandvliet, A.S. et al. Marginal increase of sunitinib exposure by grapefruit juice. Cancer Chemother Pharmacol 67, 695–703 (2011). https://doi.org/10.1007/s00280-010-1367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1367-0