Abstract
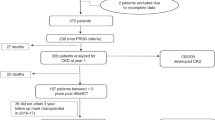
We aimed to evaluate the incidence of chronic renal injury in patients with chronic myeloid leukemia in the chronic phase (CML-CP) receiving tyrosine kinase inhibitors (TKIs) and to identify the associated factors. Data for CML-CP patients with normal estimated glomerular filtration rate (eGFR) at baseline and receiving TKI therapy ≥ 3 months were retrospectively reviewed. The CRAE (chronic renal adverse event, defined as a 30% eGFR reduction from baseline or eGFR < 60 ml/min/1.73 m2 ≥ 90 days whichever occurred first)-free survival rates at 3 years in the imatinib cohort (n = 360) were significantly lower than those in the nilotinib cohort (n = 100) (55% versus 77%, P = 0.001) as a first-line TKI therapy. In multivariate analyses, imatinib, male sex, increasing age, and previous non-TKI treatment were associated with poor CRAE-free survival. In newly diagnosed patients who received imatinib treatment (n = 40), 24-h urine protein levels significantly increased after 6 months, and urinary β2-microglobulin values significantly increased compared to those in the nilotinib cohort (n =15) at 36 months (P = 0.042) and 42 months (P = 0.039). There was no significant difference in CRAE-free survival rates at 3 years between the nilotinib (n = 65) and dasatinib (n = 74) cohorts (67% versus 83%, P = 0.832) as second- or third-line TKI therapies. In multivariate analyses, previous non-TKI treatment was associated with poor CRAE-free survival. We concluded that imatinib was significantly correlated to chronic renal injury, possibly associated with glomerulus and renal tubular injury, compared with nilotinib as a first-line TKI therapy in CML-CP patients. However, nilotinib and dasatinib had similar mild adverse impacts on renal function as second- or third-line therapies.





Similar content being viewed by others
References
Marcolino MS, Boersma E, Clementino NC, Macedo AV, Marx-Neto AD, Silva MH, van Gelder T, Akkerhuis KM, Ribeiro AL (2011) Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann Oncol 22(9):2073–2079
Yilmaz M, Lahoti A, O'Brien S, Nogueras-González GM, Burger J, Ferrajoli A, Borthakur G, Ravandi F, Pierce S, Jabbour E, Kantarjian H, Cortes JE (2015) Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer 121(21):3894–3904
Molica M, Scalzulli E, Colafigli G, Fegatelli DA, Massaro F, Latagliata R, Foà R, Breccia M (2018) Changes in estimated glomerular filtration rate in chronic myeloid leukemia patients treated front line with available TKIs and correlation with cardiovascular events. Ann Hematol 97(10):1803–1808
Hino A, Yoshida H, Tada Y, Koike M, Minami R, Masaie H, Ishikawa J (2016) Changes from imatinib mesylate to second generation tyrosine kinase inhibitors improve renal impairment with imatinib mesylate in chronic myelogenous leukemia. Int J Hematol 104(5):605–611
Cortes JE, Gambacorti-Passerini C, Kim DW, Kantarjian HM, Lipton JH, Lahoti A, Talpaz M, Matczak E, Barry E, Leip E, Brümmendorf TH, Khoury HJ (2017) Effects of bosutinib treatment on renal function in patients with Philadelphia chromosome-positive leukemias. Clin Lymphoma Myeloma Leuk 17(10):684–695
Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS (2014) Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311(24):2518–2531
Radich JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, Bhatnagar B, Curtin P, DeAngelo DJ, Gotlib J, Hobbs G, Jagasia M, Kantarjian HM, Maness L, Metheny L, Moore JO, Pallera A, Pancari P, Patnaik M, Purev E, Rose MG, Shah NP, Smith BD, Snyder DS, Sweet KL, Talpaz M, Thompson J, Yang DT, Gregory KM, Sundar H (2018) Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 16(9):1108–1135
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, Hjorth-Hansen H, Hughes TP, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Martinelli G, Mayer J, Muller MC, Niederwieser D, Pane F, Radich JP, Rousselot P, Saglio G, Saussele S, Schiffer C, Silver R, Simonsson B, Steegmann JL, Goldman JM, Hehlmann R (2013) European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 122(6):872–884
Perazella MA (2005) Drug-induced nephropathy: an update. Expert Opin Drug Saf 4(4):689–706
Choudhury D, Ahmed Z (2006) Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol 2(2):80–91
Miyata T, Jadoul M, Kurokawa K, Van Ypersele de Strihou C (1998) Beta-2 microglobulin in renal disease. J Am Soc Nephrol 9(9):1723–1735
Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295(1):139–145
Floege J, Eitner F, Alpers CE (2008) A new look at platelet derived growth factor in renal disease. J Am Soc Nephrol 19(1):12–23
Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R (2003) Activation of Src kinase in platelet derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol 163(1):277–286
Boor P, Ostendorf T, Floege J (2014) PDGF and the progression of renal disease. Nephrol Dial Transplant 29(Suppl 1):i45–i54
Kishioka H, Fukuda N, Wen-Yang H, Nakayama M, Watanabe Y, Kanmatsuse K (2001) Effects of PDGF A-chain antisense oligodeoxynucleotides on growth of cardiovascular organs in stroke-prone spontaneously hypertensive rats. Am J Hypertens 14(5 Pt 1):439–445
Zoja C, Corna D, Rottoli D, Zanchi C, Abbate M, Remuzzi G (2006) Imatinib ameliorates renal disease and survival in murine lupus autoimmune disease. Kidney Int 70(1):97–103
Doi T, Vlassara H, Kirstein M, Yamada Y, Striker GE, Striker LJ (1992) Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci U S A 89(7):2873–2877
Funding
This study was funded by the National Natural Science Foundation of China (No. 81770161).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, X., Qin, Y., Huang, X. et al. Assessment of chronic renal injury in patients with chronic myeloid leukemia in the chronic phase receiving tyrosine kinase inhibitors. Ann Hematol 98, 1627–1640 (2019). https://doi.org/10.1007/s00277-019-03690-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03690-2