Abstract
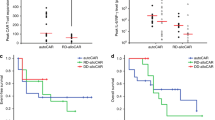
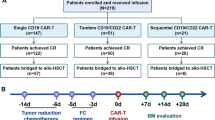
Chimeric antigen receptor modified T cells against CD19 (CART19s) have potent anti-leukemia activities in patients with refractory/relapsed acute lymphoblastic leukemia (R/R ALL). This study was designed to investigate the correlation between safety/efficacy and therapeutic modalities including chemotherapy and CART19 therapy. Total 23 and 69 patients were enrolled in the CART19 group and in the chemotherapy group, respectively. The safety and efficacy profiles of 66 and 22 patients in the 2 groups were evaluated. The complete remission (CR) rate was higher in the CART19 group than that in the chemotherapy group (90.9 vs 37.9%, P = 0.000). For patients relapsed after allo-HSCT and chemotherapy, CR rates were 100% (8/8) vs 48.0% (12/25) (P = 0.009) and 85.7% (12/14) vs 31.7% (13/41) (P = 0.000), respectively. Moreover, a higher percentage in the CART19 group had results below the threshold for minimal residual disease (100 vs 7.58%, P = 0.000). In survival analysis, the overall survival rate at 12 months was higher in the CART19 group than that in the chemotherapy group (60.9 vs 10.1%, P = 0.000). For post-transplant patients achieving CR, 25.0% (2/8) and 75.0% (9/12) complicated with GVHD (P = 0.04) in the CART19 group and chemotherapy group, respectively. For all CR patients, the median duration of absolute neutrophil count less than 500/μL and platelet count less than 20,000/μL were longer in the CART19 group than in the chemotherapy group (p = 0.0047 and 0.0003, respectively). Our data demonstrated that patients with CART19s therapy acquired higher rates of remission and longer survival, confirming the encouraging application of CART19 therapy in R/R ALL.


Similar content being viewed by others
References
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foà R, Bassan R, Arslan Ö, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Brüggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, Topp MS (2017) Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376(9):836–847. https://doi.org/10.1056/NEJMoa1609783
Gökbuget N, Kelsh M, Chia V, Advani A, Bassan R, Dombret H, Doubek M, Fielding AK, Giebel S, Haddad V, Hoelzer D, Holland C, Ifrah N, Katz A, Maniar T, Martinelli G, Morgades M, O'Brien S, Ribera JM, Rowe JM, Stein A, Topp M, Wadleigh M, Kantarjian H (2016) Blinatumomab vs historical standard therapy of adult relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J 6(9):e473. https://doi.org/10.1038/bcj.2016.84
Gökbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R et al (2016) International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 101(12):1524–1533. https://doi.org/10.3324/haematol.2016.144311
Stein A, Palmer J, Tsai NC, Al Malki MM, Aldoss I, Ali H, Aribi A, Farol L, Karanes C, Khaled S, Liu A, O'Donnell M, Parker P, Pawlowska A, Pullarkat V, Radany E, Rosenthal J, Sahebi F, Salhotra A, Sanchez JF, Schultheiss T, Spielberger R, Thomas SH, Snyder D, Nakamura R, Marcucci G, Forman SJ, Wong J (2017) Phase I trial of total marrow and lymphoid irradiation transplantation conditioning in patients with relapsed/refractory acute leukemia. Biol Blood Marrow Transplant 23(4):618–624. https://doi.org/10.1016/j.bbmt.2017.01.067
Jabbour E, Short NJ, Jorgensen JL, Yilmaz M, Ravandi F, Wang SA, Thomas DA, Khoury J, Champlin RE, Khouri I, Kebriaei P, O'Brien SM, Garcia-Manero G, Cortes JE, Sasaki K, Dinardo CD, Kadia TM, Jain N, Konopleva M, Garris R, Kantarjian HM (2017) Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer 123(2):294–302. https://doi.org/10.1002/cncr.30264
Oriol A, Vives S, Hernández-Rivas JM et al (2010) Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica 95(4):589–596. https://doi.org/10.3324/haematol.2009.014274
Haen SP, Groh C, Schumm M, Backert L, Löffler MW, Federmann B, Faul C, Dörfel D, Vogel W, Handgretinger R, Kanz L, Bethge WA (2017) Haploidentical hematopoietic cell transplantation using in vitro T cell depleted grafts as salvage therapy in patients with disease relapse after prior allogeneic transplantation. Ann Hematol 96(5):817–827. https://doi.org/10.1007/s00277-017-2941-x
Yoo SH, Koh Y, Kim DY, Lee JH, Lee JH, Lee KH, Korean Society of Blood and Marrow Transplantation et al (2017) Salvage therapy for acute chemorefractory leukemia by allogeneic stem cell transplantation: the Korean experience. Ann Hematol 96(4):605–615. https://doi.org/10.1007/s00277-017-2919-8
DasGupta RK, Marini BL, Rudoni J, Perissinotti AJ (2017) A review of CD19-targeted immunotherapies for relapsed or refractory acute lymphoblastic leukemia. J Oncol Pharm Pract 1078155217713363:107815521771336. https://doi.org/10.1177/1078155217713363
Hay KA, Turtle CJ (2017) Chimeric antigen receptor (CAR) T cells: lessons learned from targeting of CD19 in B-cell malignancies. Drugs 77(3):237–245. https://doi.org/10.1007/s40265-017-0690-8
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, Liang Z, Wei G, Cui Q, Sun J, Jiang J, Xie J, Tan Y, Ni W, Tu J, Wang J, Jin A, Zhang H, Cai Z, Xiao L, Huang H (2017) Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res 23(13):3297–3306. https://doi.org/10.1158/1078-0432.CCR-16-1799
Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C, Liang B, Luo Y, Shi J, Jin A, Xiao L, Huang H (2016) Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol 9(1):70. https://doi.org/10.1186/s13045-016-0299-5
Tan Y, Du K, Luo Y, Shi J, Cao L, Zheng Y, Zheng G, Zhao Y, Ye X, Cai Z, Huang H (2014) Superiority of preemptive donor lymphocyte infusion based on minimal residual disease in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Transfusion 54(6):1493–1500. https://doi.org/10.1111/trf.12524
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967):517–528. https://doi.org/10.1016/S0140-6736(14)61403-3
Valecha GK, Ibrahim U, Ghanem S, Asti D, Atallah JP, Terjanian T (2017) Emerging role of immunotherapy in precursor B-cell acute lymphoblastic leukemia. Expert Rev Hematol 10:1–17. https://doi.org/10.1080/17474086
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/NEJMoa1407222
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS et al (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25.1
Dai H, Wang Y, Lu X, Han W (2016) Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst 108(7). https://doi.org/10.1093/jnci/djv439
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, Lange E, Köppler H, Kiehl M, Sökler M, Schlag R, Vehling-Kaiser U, Köchling G, Plöger C, Gregor M, Plesner T, Trneny M, Fischer K, Döhner H, Kneba M, Wendtner CM, Klapper W, Kreuzer KA, Stilgenbauer S, Böttcher S, Hallek M, international group of investigators; German CLL Study Group (GCLLSG) (2016) First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 17(7):928–942. https://doi.org/10.1016/S1470-2045(16)30051-1
Skarbnik AP, Faderl S (2017) The role of combined fludarabine, cyclophosphamide and rituximab chemoimmunotherapy in chronic lymphocytic leukemia: current evidence and controversies. Ther Adv Hematol 8(3):99–105. https://doi.org/10.1177/2040620716681749
Mauro FR, Carella AM, Molica S, Paoloni F, Liberati AM, Zaja F, Belsito V, Cortellezzi A, Rizzi R, Tosi P, Spriano M, Ferretti A, Nanni M, Marinelli M, de Propris MS, Orlando SM, Vignetti M, Cuneo A, Guarini AR, Foà R (2017) Fludarabine, cyclophosphamide and lenalidomide in patients with relapsed/refractory chronic lymphocytic leukemia. A multicenter phase I-II GIMEMA trial. Leuk Lymphoma 58(7):1640–1647. https://doi.org/10.1080/10428194.2016.1258698
Canna SW, Wrobel J, Chu N, Kreiger PA, Paessler M, Behrens EM (2013) Interferon-γ mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum 65(7):1764–1775. https://doi.org/10.1002/art.37958
Chen J, Feng X, Desierto MJ, Keyvanfar K, Young NS (2015) IFN-γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood 126(24):2621–2631. https://doi.org/10.1182/blood-2015-06-652453
Smith JN, Kanwar VS, MacNamara KC (2016) Hematopoietic stem cell regulation by type I and II interferons in the pathogenesis of acquired aplastic anemia. Front Immunol 7:330
DeAngelo DJ, Stock W, Stein AS, Shustov A, Liedtke M, Schiffer CA, Vandendries E, Liau K, Ananthakrishnan R, Boni J, Laird AD, Fostvedt L, Kantarjian HM, Advani AS (2017) Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv 1(15):1167–1180. https://doi.org/10.1182/bloodadvances.2016001925
Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC et al (2015) Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 16:57e66
Frey N (2017) Cytokine release syndrome: who is at risk and how to treat. Best Pract Res Clin Haematol 30(4):336–340. https://doi.org/10.1016/j.beha.2017.09.002
Funding
This work was supported by the grants from 973 Program (2015CB964900), the Natural Science Foundation of China (81230014, 81470341, 81520108002, 81500157), and the Key Project of Science and Technology Department of Zhejiang Province (2015C03G2010091).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Supplementary Table 1
(DOCX 17 kb)
Supplementary Table 2
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Wei, G., Hu, Y., Pu, C. et al. CD19 targeted CAR-T therapy versus chemotherapy in re-induction treatment of refractory/relapsed acute lymphoblastic leukemia: results of a case-controlled study. Ann Hematol 97, 781–789 (2018). https://doi.org/10.1007/s00277-018-3246-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3246-4