Abstract
The study purpose was to report the first case of granulocyte colony-stimulating factor (G-CSF)-induced capillary leak syndrome (CLS) in which serial extravascular lung water (EVLW) measurements were performed and to compare this case with previously reported cases. To identify previously reported cases, we performed a literature search, using PubMed with the following search terms: CLS, EVLW, G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF) and stem cell transplantation and the references in the bibliographies of the papers retrieved. To obtain additional information about these cases, we contacted the authors by e-mail. We describe the case of a 68-year-old woman who developed severe CLS during G-CSF treatment after autologous haematological stem cell transplantation. CLS is caused by damage to the endothelial cells, resulting in extravasation of plasma proteins and fluid from the capillaries into the extravascular space. This is illustrated by high values of EVLW and pulmonary vascular permeability, necessitating mechanical ventilation. We found five other case reports in the literature. The white blood cell count at the onset of the CLS varied from very low (zero) to very high (90,500/μl). The symptoms began on day 5–9 of the G-CSF treatment. All patients had fever. Three patients were mechanically ventilated and four received renal replacement therapy. Two patients died. Treatment with G-CSF can induce fatal CLS. Monitoring of EVLW in patients with severe CLS may be useful to guide fluid therapy and improve oxygenation.
Similar content being viewed by others
Introduction
Capillary leak syndrome (CLS) occurs frequently in patients with septic shock, pancreatitis and multiple trauma [17]. In the haematological literature it has been reported after infusions of interleukin-2, interleukin-4, tumor necrosis factor, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) as well as after autologous and allogeneic stem cell transplantation [4, 17, 19].
The purpose of the study was to report the first case of G-CSF-induced CLS in which serial extravascular lung water (EVLW) measurements were performed and to compare this case with previously reported cases.
Methods
Extravascular lung water measurement
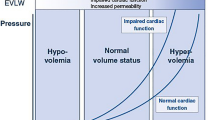
The EVLW of our patient was measured by transpulmonary thermodilution with the PiCCO system (Pulsion Medical Systems, Munich, Germany) [18]. This system includes a thermistor-tipped catheter placed in the femoral, brachial or axillary artery and a PiCCO monitor. A known volume of cold saline is injected through a central venous catheter, which induces a downstream temperature change, recorded by the arterial catheter. As a result, a thermodilution curve can be constructed. The cardiac output is determined using the Stewart-Hamilton method [15]. The product of cardiac output and mean transit time of the thermodilution curve represents the volume transversed by the cold, which is called the intrathoracic thermal volume (ITTV). The product of cardiac output and exponential downslope time of the thermodilution curve represents the largest individual mixing volume in a series of thermodilution mixing chambers, which is the pulmonary thermal volume (PTV). The ITTV consists of the PTV and the sum of the end-diastolic volumes of all cardiac chambers. Accordingly, the global end-diastolic volume (GEDV) is calculated as: GEDV = ITTV − PTV. Based on a linear relation between GEDV and intrathoracic blood volume (ITBV): ITBV = 1.25 × GEDV. The EVLW is the difference between ITTV and ITBV: EVLW = ITTV − ITBV. Pulmonary blood volume (PBV) and pulmonary vascular permeability index (PVPI) are derived from these values: PBV = ITBV − GEDV and PVPI = EVLWI/PBV. Absolute values for GEDV and ITBV are normalised as indexed by body surface area (GEDVI and ITBVI) and for EVLW by body weight (EVLWI).
Literature search
To identify previously reported cases, we performed a literature search, using PubMed with the following search terms: CLS, EVLW, G-CSF, GM-CSF and stem cell transplantation and the references in the bibliographies of the most important papers retrieved. To obtain additional information about these cases, we contacted the authors by e-mail.
Results
Case report
Two years prior to autologous haematopoietic stem cell transplantation (AHSCT), a previously well 66-year-old woman was diagnosed with stage IIIB diffuse large B-cell lymphoma. She received eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine and methylprednisolone) and achieved complete remission. She relapsed 1.5 years later and was treated with two cycles of DHAP (cisplatin, cytarabine and dexamethasone) and VIM (mitoxantrone, ifosfamide and etoposide) and achieved a second complete remission. The patient received BEAM (intrathecal methotrexate, hydrocortisone and cytarabine on day −6, carmustine on day −5, etoposide and cytarabine from day −5 to day −2 and melphalan on day −2) followed by infusion of peripheral blood stem cells (PBSC) containing 6.877×106/kg CD34+ progenitor cells (day 0). G-CSF (480 μg, 6.49 μg/kg, 265.19 μg/m2) was administered subcutaneously every day starting on day 0 and was stopped on day 11 because the absolute neutrophil count (ANC) exceeded 500/μl.
On day 2 after AHSCT the blood urea nitrogen (BUN) started to increase, as well as body weight on day 4 and creatinine on day 5. Furosemide up to a dose of 250 mg had no effect and the urinary output decreased to 240 ml per day. She became somnolent and developed lower limb oedema. The patient was admitted to the medical intensive care unit (ICU) on day 11. She had a blood pressure of 98/45 mmHg, the pulse was 137, the central venous pressure 14 mmHg, the respiratory rate 20, the temperature 38.4°C (1st day of fever) and the Glasgow coma scale 15/15. We noticed crackles in both lung bases. The laboratory values are summarised in Table 1. Chest X-ray showed marked pulmonary congestion.
Slow extended daily dialysis (SLEDD) was started on day 12. On day 13, the bilateral pulmonary infiltrates worsened and pleural effusions emerged. We treated her with norepinephrine and dobutamine. She became more hypoxic and needed noninvasive bi-level positive airway pressure (BiPAP) ventilation. Because of respiratory acidosis and worsening hypoxia, she was intubated and mechanically ventilated on day 14. On day 16, the liver function tests deteriorated and she became icteric. Hepatomegaly and ascites were absent. The hepatic venous pressure gradient was normal. Transjugular liver biopsy revealed drug-induced hepatitis without signs of veno-occlusive disease.
On day 21, SLEDD was switched to continuous venovenous haemofiltration (CVVH) and corticosteroids were initiated (hydrocortisone 100 mg 3 times daily). Volume measurements were performed by transpulmonary thermodilution with a 5F Pulsiocath catheter and a PiCCO monitor (Pulsion Medical Systems, Munich, Germany) [(Fig. 1). GEDVs were in the low normal range while the EVLWI was increased. Despite ultrafiltration rates of 3000–4500 ml per day, the cumulative fluid balance remained positive, and there was rapidly worsening generalised oedema (Fig. 2 panel a). The corticosteroids had only a temporary effect on the EVLWI and the pulmonary infiltrates persisted (Fig. 2 panel b). Figure 3 shows the evolution of body weight and capillary leak index (CLI). Finally, hypoxia, respiratory and metabolic acidosis and haemodynamic instability worsened and the patient died on day 40. All cultures (repeated cultures of sputum, endobronchial aspiration, blood and urine), including for opportunistic pathogens, remained negative. The Aspergillus antigen index was repeatedly measured and always within the normal range.
Evolution of the PiCCO measurements of extravascular lung water index (EVLWI, estimated normal range: 3–7 ml/kg), global end-diastolic volume index (GEDVI, estimated normal range: 680–800 ml/m2) and pulmonary vascular permeability index (PVPI, estimated normal range: 1–2) from day 15 to day 28. EVLWI and PVPI were always high, with EVLWI peaking at 15 ml/kg. The GEDVI decreased to a minimum of 696 ml/m2. Corticosteroids were started on day 21 and had a temporary beneficial effect on EVLWI and PVPI.
Literature search
We identified five publications that described seven patients with G-CSF-induced CLS [3, 4, 7, 13, 17]. Of these, we could find four full-text articles on the internet or in Belgian libraries [3, 4, 7, 17]. To obtain additional information about these cases, we contacted the authors or their hospitals by e-mail and received an answer from two [4, 17]. The findings in our case and the previously published cases are summarised in Table 2.
The white blood cell count at the onset of the CLS varied from very low (zero) to very high (90,500/μl). One of the six patients was a peripheral blood progenitor cell donor who had no underlying disease and did not receive chemotherapy [3]. The symptoms began on day 5–9 of the G-CSF treatment. All patients had fever. Three patients were mechanically ventilated and four received renal replacement therapy. Two patients died.
Discussion
CLS is arbitrarily defined in the currently available literature. Clinical and biochemical findings include generalised oedema (peripheral and pulmonary oedema, ascites, pleural and pericardial effusion), weight gain, hypotension, prerenal failure, multiple organ failure and hypoalbuminaemia [3, 17, 20]. Fever was present in all of the reported cases of G-CSF-induced CLS. CLS is caused by damage to the endothelial cells, resulting in extravasation of plasma proteins and fluid from the capillaries into the extravascular space [20]. Despite generalised oedema, vigorous fluid therapy is necessary to compensate for intravascular volume depletion. This will further expand the extravascular space, as is illustrated in our patient by an increasing cumulative fluid balance and a persistently high EVLWI (and PVPI), despite CVVH with aggressive ultrafiltration. Three of the six reported patients were mechanically ventilated. Two patients died. One of the previously reported cases illustrates that neither an underlying haematological illness nor chemotherapy are required for this endothelial damage [3].
Fluid management effects the development and resolution of cardiogenic and noncardiogenic pulmonary oedema. Mitchell et al. have demonstrated that a protocol using measurements of EVLW instead of the pulmonary artery occlusion pressure to guide fluid management in mixed ICU patients requiring pulmonary artery catheterisation was associated with reduced ventilator and ICU days and a lower cumulative fluid balance [11]. This may also be the case in CLS. The latter investigators administered vasopressors instead of fluids when patients with an EVLWI of greater than 7 ml/kg developed hypotension. This approach was associated with a small but significant increase in creatinine and BUN.
The literature suggests two hypotheses about the pathogenesis of G-CSF-induced CLS, stressing either the role of granulocytes or a direct effect of G-CSF. Heitger et al. reported a case of G-CSF-induced CLS with an onset 36 h after cessation of G-CSF, arguing against a direct effect of G-CSF on the capillary endothelium. However, when the CLS became evident, there was a marked increase in the ANC [7]. In fact, CLS can also occur in other situations of highly stimulated granulopoiesis, for example in the engraftment syndrome [1, 9, 19]. Post-transplant G-CSF increased the incidence of the engraftment syndrome in one [8] but not conclusively in other series [5, 12, 16]. Recombinant human G-CSF (filgrastim) increases the neutrophil count and enhances their phagocytic and cytotoxic functions as well as their expression of adhesion molecules [14]. The other myeloid growth factor, GM-CSF, has been shown to cause enhanced granulocyte aggregability and adhesiveness, resulting in the endothelial sticking phenomenon. This consists of sequestration of granulocytes in the lung microvasculature, formation of granulocyte aggregates, and activation of the complement cascade [6]. The same might be applicable to G-CSF.
However, Dagdemir et al. reported a patient who developed CLS twice while receiving G-CSF, without any time relationship between the onset of symptoms and the rapid increase of the white blood cell count [4]. According to the authors, a direct effect of G-CSF on the endothelium may have played the major role. They emphasised that G-CSF is able to cause CLS even when the white blood cell count is very low. On the other hand, a low white blood cell count at the onset of symptoms could also result from the endothelial sticking phenomenon with granulocyte sequestration, as was stated in a report of CLS induced by GM-CSF [2]. In the future, differential cell count of the bronchoalveolar lavage fluid might provide us with more information on the pulmonary sequestration of granulocytes.
Treatment of CLS with corticosteroids may be beneficial, presumably by interfering with granulocyte function and cytokine release [3, 4, 17]. Corticosteroids had a temporary beneficial effect on the EVLWI and PVPI in our patient. Importantly, none of the reported patients had proven sepsis.
We recently found that a novel parameter called capillary leak index [CLI = CRP level (mg/l) divided by albumin level (g/l)], calculated within 24 h after ICU admission, was an independent predictor for mortality in mixed ICU patients. A high CLI was correlated with organ failure [10]. In our patient, the CLI reached its highest value on day 21 and decreased after the administration of corticosteroids.
In conclusion, treatment with G-CSF can induce fatal CLS. Monitoring of EVLW in patients with severe CLS may be useful to guide fluid therapy and improve oxygenation.
References
Akasheh M, Eastwood D, Vesole DH (2003) Engraftment syndrome after autologous hematopoietic stem cell transplant supported by granulocyte colony-stimulating factor (G-CSF) versus granulocyte-macrophage colony-stimulating factor (GM-CSF). Bone Marrow Transplant 31:113–116
Arning M, Kliche KO, Schneider W (1991) GM-CSF therapy and capillary-leak syndrome. Ann Hematol 62:83
de Azevedo AM, Goldberg Tabak D (2001) Life-threatening capillary leak syndrome after G-CSF mobilization and collection of peripheral blood progenitor cells for allogeneic transplantation. Bone Marrow Transplant 28:311–312
Dagdemir A, Albayrak D, Dilber C, Totan M (2001) G-CSF related capillary leak syndrome in a child with leukemia. Leuk Lymphoma 42:1445–1447
Edenfield WJ, Moores LK, Goodwin G, Lee N (2000) An engraftment syndrome in autologous stem cell transplantation related to mononuclear cell dose. Bone Marrow Transplant 25:405–409
Emminger W, Emminger-Schmidmeier W, Peters C, Susani M, Hawliczek R, Hocker P, Gadner H (1990) Capillary leak syndrome during low dose granulocyte-macrophage colony-stimulating factor (rh GM-CSF) treatment of a patient in a continuous febrile state. Blut 61:219–221
Heitger A, Maurer K, Neu N, Fink FM (1998) Capillary leak syndrome in a patient with septicemia and granulocyte-colony-stimulating factor (G-CSF)-induced accelerated granulopoiesis. Med Pediatr Oncol 31:126–127
Lee CK, Gingrich RD, Hohl RJ, Ajram KA (1995) Engraftment syndrome in autologous bone marrow and peripheral stem cell transplantation. Bone Marrow Transplant 16:175–182
Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M (2003) Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone Marrow Transplant 31:393–397
Malbrain MLNG, Debaveye Y, De Coninck J, Derlmarcelle D (2001) Capillary leakage index as outcome predictor (abstract)? Intensive Care Med 27 [Suppl 2]:S229
Mitchell JP, Schuller D, Calandrino FS, Schuster DP (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145:990–998
Moreb JS, Kubilis PS, Mullins DL, Myers L, Youngblood M, Hutcheson C (1997) Increased frequency of autoaggression syndrome associated with autologous stem cell transplantation in breast cancer patients. Bone Marrow Transplant 19:101–106
Oeda E, Shinohara K, Kamei S, Nomiyama J, Inoue H (1994) Capillary leak syndrome likely the result of granulocyte colony-stimulating factor after high-dose chemotherapy. Intern Med 33:115–119
Ohsaka A, Kitagawa S, Sakamoto S, Miura Y, Takanashi N, Takaku F, Saito M (1989) In vivo activation of human neutrophil functions by administration of recombinant human granulocyte colony-stimulating factor in patients with malignant lymphoma. Blood 74:2743–2748
Pfeiffer UJ, Backus G, Blümel G, Eckart J, Müller P, Winkler P, Zeravik J, Zimermann GJ (1990) A fiberoptics-based system for integrated monitoring of cardiac output, intrathoracic blood volume, extravascular lung water, O2 saturation, and a–v differences. In: Lewis FR, Pfeiffer UJ (eds) Practical applications of fiberoptics in critical care monitoring. Springer, Berlin Heidelberg New York, pp 114–125
Ravoet C, Feremans W, Husson B, Majois F, Kentos A, Lambermont M, Wallef G, Capel P, Beauduin M, Delannoy A (1996) Clinical evidence for an engraftment syndrome associated with early and steep neutrophil recovery after autologous blood stem cell transplantation. Bone Marrow Transplant 18:943–947
Rechner I, Brito-Babapulle F, Fielden J (2003) Systemic capillary leak syndrome after granulocyte colony-stimulating factor (G-CSF). Hematol J 4:54–56
Sakka SG, Ruhl CC, Pfeiffer UJ, Beale R, McLuckie A, Reinhart K, Meier-Hellmann A (2000) Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med 26:180–187
Spitzer TR (2001) Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 27:893–898
Vial T, Descotes J (1995) Clinical toxicity of cytokines used as haemopoietic growth factors. Drug Saf 13:371–406
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deeren, D.H., Zachee, P. & Malbrain, M.L.N.G. Granulocyte colony-stimulating factor-induced capillary leak syndrome confirmed by extravascular lung water measurements. Ann Hematol 84, 89–94 (2005). https://doi.org/10.1007/s00277-004-0946-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-004-0946-8