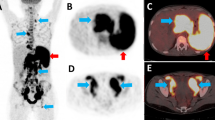
Abstract
Purpose
To determine the diagnostic accuracy of a semiautomated 18F-FDG PET/CT measurement of total lesion glycolysis (TLG), maximum and peak standardized uptake value at lean body mass (SUL-Max and SUL-Peak), qualitative estimates of left/right nodal symmetry and FDG uptake for differentiating lymphoma from reactive adenopathy in HIV-infected patients.
Methods
We retrospectively analyzed 41 whole-body 18F-FDG PET/CT studies performed in HIV-infected patients for clinical reasons. The study received institutional review board approval. Of the 41 patients, 19 had biopsy-proven untreated lymphoma, and 22 with reactive adenopathy without malignancy on follow-up were used as controls. Nodal and extranodal visual qualitative metabolic scores, SUL-Max, SUL-Peak, CT nodal size, and PERCIST 1.0 threshold-based TLG and metabolic tumor volume (MTV) were determined. The qualitative intensity of nodal involvement and symmetry of uptake were compared using receiver operator curve (ROC) analysis. HIV plasma viral RNA measurements were also obtained.
Results
All of the quantitative PET metrics performed well in differentiating lymphoma from reactive adenopathy and performed better than qualitative visual intensity scores. The areas under the ROC curves (AUC) were significantly higher for TLG = 0.96, single SUL-Peak = 0.96, single SUL-Max = 0.97, and MTV = 0.96, compared to 0.67 for CT nodal size (p < 0.001). These PET metrics performed best in separating the two populations in aviremic patients, with AUCs of 1 (AUC 0.91 for CT nodal size). TLG, MTV, SUL-Peak and SUL-Max were more reliable markers among viremic individuals, with AUCs between 0.84 and 0.93, compared to other metrics. PET metrics were significantly correlated with plasma viral load in HIV-reactive adenopathy controls. Asymmetrical FDG uptake had an accuracy of 90.4 % for differentiating lymphoma from reactive adenopathy in HIV-infected patients.
Conclusion
Quantitative PET metabolic metrics as well as the qualitative assessment of symmetry of nodal uptake appear to be valuable tools for differentiating lymphoma from reactive adenopathy in HIV-infected patients using FDG PET. These parameters appear more robust in aviremic patients.





Similar content being viewed by others
References
Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223–48.
Beral V, Peterman T, Berkelman R, Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337:805–9.
Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:14–9.
Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–62.
Scharko AM, Perlman SB, Pyzalski RW, Graziano FM, Sosman J, Pauza CD. Whole-body positron emission tomography in patients with HIV-1 infection. Lancet. 2003;362:959–61.
Liu Y. Demonstrations of AIDS-associated malignancies and infections at FDG PET-CT. Ann Nucl Med. 2011;25:536–46.
Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–68.
Keidar Z, Gurman-Balbir A, Gaitini D, Israel O. Fever of unknown origin: the role of 18F-FDG PET/CT. J Nucl Med. 2008;49:1980–5.
Kouijzer IJ, Bleeker-Rovers CP, Oyen WJ. FDG-PET in fever of unknown origin. Semin Nucl Med. 2013;43:333–9.
Bental M, Deutsch C. Metabolic changes in activated T cells: an NMR study of human peripheral blood lymphocytes. Magn Reson Med. 1993;29:317–26.
Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–9.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8.
O’Doherty MJ, Barrington SF, Campbell M, Lowe J, Bradbeer CS. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997;38:1575–83.
Goudarzi B, Jacene HA, Wahl RL. Measuring the “unmeasurable”: assessment of bone marrow response to therapy using FDG-PET in patients with lymphoma. Acad Radiol. 2010;17(9):1175–85.
Lodge MA, Chaudhry MA, Udall DN, Wahl RL. Characterization of a perirectal artifact in 18F-FDG PET/CT. J Nucl Med. 2010;51(10):1501–6.
Liu Y. Concurrent FDG avid nasopharyngeal lesion and generalized lymphadenopathy on PET-CT imaging is indicative of lymphoma in patients with HIV infection. AIDS Res Treat. 2012; 2012:764291.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50.
Frisch M, Biggar RJ, Engels EA, Goedert JJ, AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45.
Aldous JL, Haubrich RH. Defining treatment failure in resource-rich settings. Curr Opin HIV AIDS. 2009;4:459–66.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Scadden DT. AIDS-related malignancies. Annu Rev Med. 2003;54:285–303.
Song MK, Chung JS, Shin HJ, Moon JH, Lee JO, Lee HS, et al. Prognostic value of metabolic tumor volume on PET/CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci. 2012;103:477–82.
Iyengar S, Chin B, Margolick JB, Sabundayo BP, Schwartz DH. Anatomical loci of HIV-associated immune activation and association with viraemia. Lancet. 2003;362:945–50.
Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B, Levine AM. AIDS-related Burkitt’s lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. J Clin Oncol. 2005;23:4430–8.
Brust D, Polis M, Davey R, Hahn B, Bacharach S, Whatley M, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS. 2006;20:985–93.
Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies an international perspective. Hematol Oncol Clin North Am. 2003;17:673–96.
Goshen E, Davidson T, Avigdor A, Zwas TS, Levy I. PET/CT in the evaluation of lymphoma in patients with HIV-1 with suppressed viral loads. Clin Nucl Med. 2008;33:610–4.
Lucignani G, Orunesu E, Cesari M, Marzo K, Pacei M, Bechi G, et al. FDG-PET imaging in HIV-infected subjects: relation with therapy and immunovirological variables. Eur J Nucl Med Mol Imaging. 2009;36:640–7.
Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of (18)F-FDG: standardized uptake values in normal tissues. J Nucl Med. 2004;45:784–8.
Acknowledgments
We thank Dr. Richard Ambinder for his contribution, which included experimental design and thoughts on clinical relevance. We also thank Judy Buchannan for helping edit and improve the manuscript. The work was supported by a Quantitative Imaging Network grant awarded by the National Cancer Institute (NCI-U-01-CA 140204).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
A related editorial commentary can be found at doi 10.1007/s00259-014-2701-2
Rights and permissions
About this article
Cite this article
Mhlanga, J.C., Durand, D., Tsai, HL. et al. Differentiation of HIV-associated lymphoma from HIV-associated reactive adenopathy using quantitative FDG PET and symmetry. Eur J Nucl Med Mol Imaging 41, 596–604 (2014). https://doi.org/10.1007/s00259-013-2671-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2671-9