Abstract
Introduction
Esthesioneuroblastoma (ENB) is an aggressive neuroectodermal malignancy in the upper nasal cavity with local infiltration and lymphatic or hematogenous metastasis. The purpose of this paper is to document three types of direct intracranial extensions by ENB using computed tomography (CT) and magnetic resonance imaging (MRI).
Methods
Eleven patients with pathologically confirmed ENB were admitted in our hospital between December 2002 and December 2008. Their magnetic resonance (MR; n = 10) and CT (n = 8) images were retrospectively reviewed, and particular attention was paid to tumor location and extension, enhancement pattern, cervical lymph node metastasis, and Kadish stage.
Results
The majority of patients were male (8/11) with Kadish stage C tumor (10/11). Three types of direct intracranial extension by ENBs were put forward according to their MR and CT findings. The primary tumors were well-defined soft-tissue masses centered in the roof of the nasal cavity eroding into the paranasal sinuses (11/11), the contralateral nasal cavity (4/11), the cranial cavity (5/11), and the fossa orbitalis (3/11). The tumor parenchyma were hypointensity on T1-weighted images, heterogeneous hyperintensity on T2-weighted images, and isodensity or slight hyperdensity on CT images with scattered necroses (4/11) and marginal cysts(4/11). Their enhancements were significant and inhomogeneous. Cervical lymph nodes metastases were observed in four patients (4/11), but no pathologically proved distant metastasis was observed.
Conclusion
Three types of direct intracranial extensions by ENB can be found on CT and MRI: cranio-orbital-nasal-communicating ENB, cranio-nasal-communicating ENB, and orbital-nasal-communicating ENB.
Similar content being viewed by others
Introduction
Esthesioneuroblastoma (ENB) is a rare, aggressive neuroectodermal malignancy of the upper nasal cavity [1–4]. It accounts for 3–5% [5–9] of all nasal malignant tumors, and its incidence is estimated at 0.4 per million [10]. Since it was first described by Berger et al. in 1924 [11], controversy has been surrounding its exact cytopathologic origin [12] and classification [13–17]. Recently, immunohistochemical and histological examinations imply that ENB is of neuroendocrine cell origin and is classified as a subtype of olfactory neuroblastomas (ONBs). ONBs are uncommon neuroectodermal malignancies assumed to arise from olfactory receptor cells high in the nasal cavity and are generally considered to be APUDomas. Other proposed histological subtypes of ONBs include olfactory neuroepithelioma (esthesioneuroepithelioma) and esthesioneurocytoma differing from ENB in morphological appearance.
ENB has a propensity of locally infiltrating into the paranasal sinuses, brain, and sometimes the orbit [16]. Resection and radiotherapeutic techniques, similar to those applied for paranasal sinus tumors, thus, risk damaging these critical structures and result in late sequelae [18, 19]. Furthermore, it has the probability of disseminating to the cervical lymph nodes and rarely other distant organs. Hence, optimistic management of an ENB patient depends in a large degree on accurate tumor locating and staging. To date, magnetic resonance imaging (MRI) and computed tomography (CT) are the mainstream cross-sectional imaging modalities for its evaluation [20]. Till now, more than 1,000 cases have been reported [16, 21–25], including a meta-analysis of 390 cases from publications between 1990 and 2000 [2], but most of them are single case reports focusing on the histogenetic, pathologic, and clinical characteristics. However, data of its imaging characteristics are still scarce. Therefore, this paper mainly documents the magnetic resonance (MR) and CT findings of 11 pathologically confirmed ENBs, aiming to increase the dataset and evaluate the application of MRI and CT in its diagnosing and staging. Besides, three types of direct intracranial extension by ENB are put forward, which have not been proposed before, as far as we know.
Materials and methods
Patients
From December 2002 to December 2008, 11 patients (eight males and three females; 11 ~ 59 years old; mean age, 44 years) with pathologic diagnosis of ENB were admitted in our hospital. Ten of them took pretreatment MR examination and eight underwent pretreatment and/or post-treatment CT scanning of the brain and sinonasal area (seven patients had both modalities).
Imaging techniques
All MR scans were performed on a 1.5-T system (Magnetom vision plus; Siemens, Erlangen, Germany) using a standard quadrature head coil (field of view, 22 cm; slice thickness, 5 ~ 8 mm with 0.1-mm interslice gap; and matrix size, 256 × 224). Preenhanced T1-weighted spin-echo images (repetition time/echo time (TR/TE), 450–700/8–12 ms) and T2-weighted spin-echo images (TR/TE, 4,500–5,500/80–100 ms) were obtained. Fluid-attenuated inversion recovery sequence images (FLAIR; TR/TE, 8,000/120 ms; inversion time, 110 ms) were available in two cases. After the intravenous administration of gadopentetate dimeglumine (Magnevist; Bayer Schering, Berlin, Germany; 0.1–0.2 mmol/kg (body weight) b.w.), T1-weighted spin-echo images were obtained in the axial, coronal, and sagittal plane.
All CT scans were performed with a Somatom Plus 4 scanner (Siemens, Enlargen, Germany) with contiguous 3-mm-thick axial slices before and after the intravenous administration of contrast agent (Omnipaque 300 mg I/ml; 1.5 ~ 2.0 ml/kg b.w.; injection rate, 2.0 ~ 2.5 ml/s).
Image analysis
Each MR and CT image was jointly reviewed by two experienced radiologists retrospectively blind to the histological diagnosis and reached decisions by consensus. Images were assessed mainly for tumor location and local extension, signal and density, enhancement pattern, as well as lymph-node metastasis. The primary clinical stages were classified as A, B, and C, according to the criteria established by Kadish et al. [26]: stage A tumors are limited to the nasal cavity, stage B tumors involve the nasal cavity and paranasal sinuses, and stage C tumors extend beyond these structures.
Results
The MR and CT findings of the 11 ENB patients together with their clinical data are detailed in Table 1. The majority of patients presented were male (8/11) with Kadish stage C disease (10/11).
On MR and CT images, all 11 cases exhibited well-defined soft-tissue masses within the unilateral (10/11) or bilateral (1/11) nasal cavity (centered in the superior nasal meatus and cribriform plate) with adjacent structure invasion, including one cranio-orbital-nasal-communicating tumor (Fig. 1), four cranio-nasal-communicating tumors (Figs. 2, 3, 4) and two orbital-nasal-communicating tumors. Compared with the gray matter, the tumor parenchyma appeared as hypointensity on T1-weighted images, hyperintensity on T2-weighted images, and isodensity or slight hyperdensity on precontrast CT images. Contrast enhancement was usually significant and inhomogeneous with multiple necrotic and cystic areas. Scattered intratumoral necroses (Figs. 1 and 3) were observed in four cases with complicated hemorrhage (Fig. 1) in two. And all five intracranial tumors exhibited mild peritumoral edema (Figs. 1, 2, 3); four of which had marginal cystic components (Figs. 1, 2). Besides, obliterative paranasal sinusitis was found in ten cases (10/11). Moreover, enlarged cervical lymph nodes (>1 cm in short diameter) were observed in four patients (4/11; Fig. 4c) and proved to be tumor metastases. Skin involvement was found in one patient (1/11). Ovary and liver problems were found in two patients (2/11), but they are not pathologically proved.
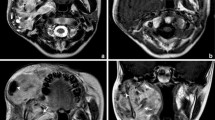
a–i (Case 2) Cranio-orbital-nasal-communicating ENB in a 15-year-old boy (brain parenchyma type). MR images show a high-aggressive tumor centered in the roof of the left nasal cavity penetrating into bilateral fossa orbitalis, left frontal lobe, and frontal sinus. Compared with the gray matter, the tumor parenchyma appear as hyperintensity on T2-weighted images (a–c) and FLAIR image (d), hypointensity on T1-weighted image (e). FLAIR image (d) exhibits the peritumoral edema more clearly, but gadolinium-enhanced T1-weighted images (f–i) visualize the tumor boundary best due to significant enhancement of all involved soft tissues. Multiple cysts (arrowheads) along the intracranial tumor margin are portrayed clearly. The necrotic component (short arrows) and complicated intratumoral hemorrhage (long arrow) can be identified
a–i (Case 11) Cranio-nasal-communicating ENB in a 59-year-old male (brain parenchyma type). MR and CT images show that the tumor located high in the left nasal cavity extends through the left cribriform plate and invades the ipsilateral frontal lobe. The tumor parenchyma is hyperintense to gray matter on T2-weighted image (a) and hypointense on T1-weighted images (b, c) with remarkable enhancement on gadolinium-enhanced T1-weighted images (d–g). A cyst (short arrows) along the margin of the intracranial tumor is portrayed clearly. Precontrast coronal CT image (h) shows an isodensity endonasal mass with slight high density to the surrounding soft tissue. Postcontrast coronal CT image (i) shows that the tumor enhances significantly and inhomogeneously. Erosion of the ethmoid labyrinth and left cribriform plate is well demonstrated (long arrows), although the margin and cyst of the tumor can not be seen as clearly as that on MRI
a–f (Case 6) Cranio-nasal-communicating ENB in a 44-year-old male (brain parenchyma type). MR images reveal a well-defined mass with multiple intratumoral necroses (short arrows) in the right upper nasal cavity and frontal lobe. Although the bulk of the tumor lies intracranially, it is still attached to the cribriform plate (long arrows). The tumor parenchyma is hyperintense to gray matter on T2-weighted image (a) and hypointense on T1-weighted image (b) with remarkable enhancement on gadolinium-enhanced T1-weighted images (c–e). No intratumoral cyst is observed. Photomicrograph (f) shows that the tumor consists of uniform, small, round-to-oval blue cells with coarsely granular chromatin, multiple small nucleoli, prominent nuclear membranes, and scant cytoplasm (hematoxylin–eosin, original magnification ×400)
a–c (Case 1) ENB in an 11-year-old girl (leptomeninges type). Postcontrast axial CT images (a–c) show a locally lamellar enhancement lining the surface of bilateral frontal lobe suggesting leptomeninges involvement. Besides, thickened craniofacial soft tissue (short arrows) and intradermal nodules (long arrows) can be found. The enhancing nodule (1 cm in short diameter) over the left submaxillary region indicates a focally enlarged lymph node (arrowhead)
a–d (Case 4) ENB in a 38-year-old female (base-of-skull type). Gadolinium-enhanced T1-weighted MR images show a well-defined tumor mass within the superior and middle nasal meatus. The axial MR image (a) shows an enhancing round signal (arrowhead) in the gyrus rectus mimicking an intracranial tumor. Nevertheless, coronary (b) and sagittal (c) MR images show bulge of the dura (short arrows). Ten days later, the operation confirmed that the ethmoid plate was destroyed by the tumor and that the cerebral dura mater was exposed, but the brain parenchyma had not been encroached by then. Posttreatment sagittal CT image (d) depicts the destruction of the left ethmoid plate directly
Discussion
ENB is an uncommon (3–5% of all nasal tumors) and aggressive malignant neurogenic tumor located high in the nasal cavity. It can affect all age groups with some researches reporting a bimodal age distribution [27] peaking first between 11 and 20 years and later from 41 to 60 years of age [28] and others; a single peak in the fifth [22, 29] or sixth [30] decade. Our patient group with age ranging between 11 and 59 years old (mean, 44 years) was basically consistent with the former. There is still a lack of consensus over the incidence of both genders. Some reported that ENB affect male and female patients equally [13]; some thought that it is slightly more common in males [16, 18, 30, 31] or otherwise [32]. Our study exhibited a male predominance (eight males versus three females), although the patient series was a bit small.
In general, the clinical symptoms of ENB were not specific and related to tumor sites and invasion [27]. It was reported that the commonest presenting symptoms were unilateral nasal obstruction, epistaxis, and anosmia [2, 23, 25, 33–39]. In our study, nine patients (82%) were presented with nasal obstruction and epistaxis lasting from 3 days to 4 years (mean, 1 month), and they, as were shown on MR images (Figs. 1, 2, 5), all developed masses in the superior and middle nasal meatus with or without the inferior nasal meatus involvement. The other two patients (18%) presented with headache (without nasal obstruction or epistaxis) had neoplasma in the superior nasal meatus without the middle and inferior nasal meatus involvement (Fig. 3). Hence, nasal obstruction and epistaxis might suggest blockage and encroachment of the superior and middle nasal meatus. Hyposmia (5/6) or anosmia (1/6) suggests involvement of either unilateral or bilateral olfactory placode. Encroachment of the brain leads to headache and/or unsteadiness (6/11; Figs. 1, 2, 3). Orbital extension (3/11; Fig. 1) caused ophthalmological symptoms such as ophthalmalgia, exophthalmos, epiphora, visual impairment, or limitation of ocular movement. Rare case reports of ear ache, tympanitis [40], and Cushing's syndrome [41, 42] or SiADH [14, 43] have been published; unfortunately, they were not found in our present patient series. Owing to its nonspecific symptoms and slow-growing nature, ENB patients often have a long history of progressive symptomatology for months, sometimes years, prior to diagnosis [2]. The average duration from symptom onset to management is 1 month (3 days–4 years) in our study and 6 months (0–18 months) in the USA, reportedly [16]. The fact that most of our patients presented with advanced Kadish stage C disease (10/11) is in support of this point of view.
Our study confirmed that MRI and CT appearance of ENB are nonspecific, sharing similar signal and density features, and enhancement patterns with other nasal neoplasms [2, 33]. The predominant site of primary tumor is the roof of the unilateral nasal cavity in the region of the cribriform plate, or laterally above the middle turbinate. Uncommon sites of initial occurrence such as the sellar and parasellar region, nasopharynx, maxillary, and sphenoid sinuses were not found in our study [44–47]. Locally, ENB extended laterally and upwards to the paranasal sinuses (n = 11; Figs. 1, 2, 5) or medially to the contralateral nasal cavity (n = 4; Figs. 1, 2), and even into the cranial cavity (n = 10; Figs. 1, 2, 3, 4, 5) or fossa orbitalis (n = 3; Fig. 1). Furthermore, ENB can also metastasize by lymphatic and hematogenic routes. It is reported that approximately 5% of patients have cervical lymph node metastasis, with a cumulative cervical metastases rate of 25% or so [28, 29], and approximately 10–30% of patients will develop distant metastases to the central nervous system (about 20–30%), lung, liver, skin, eye, bone, and parotid [9, 33, 36, 37, 39, 48]. In our study, lymph nodes metastases (ranging from 1.0 to 3.0 cm in minimal diameter) were noticed in four patients (36%) over the parapharyngeal space, the upper cervical, and submaxillary region (Fig. 4c). In addition, the 11-year-old girl with craniofacial skin involvement (Fig. 4) underwent abdominal ultrasound examination revealing a soft-tissue lump within the right cavitas pelvis which might suggest distant metastasis to the right ovary although not pathologically confirmed. Also, a 54-year-old man's abdominal ultrasound examination demonstrated a low echo-level mass in the left lobe of the liver.
This report mainly concentrates on the ten cases with direct intracranial extension and categorizes them into three types based on the depth of invasion: brain parenchyma type (5/10; Figs. 1, 2, 3), leptomeninges type (1/10; Fig. 4), and base-of-skull type (4/10; Fig. 5). The brain parenchyma type had the primary tumor extend across the cribriform plate, grow through the dura, and then infiltrate into the anterior frontal lobe. Even though the bulk of the tumor may lie intracranial, it is still attached to the cribriform plate (Fig. 3). It is reported that the presence of cysts along the intracranial tumor margin [49] and intralesional calcification [50, 51] can be considered pathognomonic for ENB. In our study, marginal cysts were observed in four of five intracranial tumor masses (Figs. 1, 2) while no definite intralesional calcification was detected. The leptomeninges type was found only in one patient showing a locally lamellar enhancement lining the brain surface (Fig. 4a), which may indicate diffuse seeding of the dural and subdural spaces. The base-of-skull type was revealed in four cases, where bulging duramatral were demonstrated on MRI (Fig. 5b, c), and thinned or destroyed cribriform complex were discovered during operation. In cases in which CT scanning reveals nasal roof erosion evidence of intracranial extension (Fig. 5d), Oskouian et al. [19] recommend acquiring MR images because they provide a more sensitive assessment for unrecognized intracranial lesions. From our experience, whether the dura is invaded or not can be determined preoperatively via carefully assessing the coronal and sagittal MR images. And the present of cerebral edema may imply the existence of intracranial extension, especially when the patient complains about either unsteadiness or headache.
Due to the high aggressive behavior and recurrence rate [23, 37] of ENB, early diagnosis, accurate staging, and close follow-ups are essential for optimistic patient management [22]. In these aspects, both MR and CT scanning are helpful in providing necessary information as to the tumor location and extension and proximity to the adjacent structures. Specifically, MRI is more accurate in depicting the exact margins of intracranial and intraorbital tumor extension on account of its multiplanar display and superior tissue contrast. And CT, especially coronal CT scanning, is excellent in evaluating the encroachment of lamina papyracea, cribriform plate, and anterior cranial base, thanks to its sensitivity to osseous structure. In Kadish stage C disease, it is important to perform MRI, especially gadolinium-enhanced T1-weighted MR imaging, to obtain essential additional information, particularly when walls of the bones are thinned or eroded. By and large, MRI is the imaging method of choice for demonstration of ENB, especially for identifying intracranial and intraorbital extension. And CT scanning can supplement MRI in further depicting bony involvement. Further studies on the management and prognosis will be summarized in our next study.
Conclusions
Three types of direct intracranial extensions by ENB can be found on CT and MRI: cranio-orbital-nasal-communicating ENB, cranio-nasal communicating ENB, and orbital-nasal-communicating ENB. Although ENB exhibits no specific MR and CT characteristics, the combined use of MR and CT techniques is excellent in providing necessary information, such as the extent of tumor and the involvement of cervical lymph nodes, which are of vital importance for treatment planning and assessing. According to our study, if the images reveal upper nasal cavity-centered polypoid mass invading the paranasal sinuses, anterior cranial fossa, or the lamina erbitalis plus enlarged cervical lymph nodes, ENB should be therefore listed in the differential diagnoses, especially when the patient complains of persistent nasal obstruction, recurrent epistaxis, hyposmia, or even anosmia. Further studies are still necessary to correlate the image findings with the surgical findings of this high aggressive malignancy.
References
Porter AB, Bernold DM, Giannini C et al (2008) Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J Neuro-oncol 90:201–204
Dulguerov P, Allal AS, Calcaterra TC (2001) Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol 2:683–690
Lee L, Le QT, Xing L (2008) Retrospective IMRT dose reconstruction based on cone-beam CT and MLC log-file. Int J Radiat Oncol Biol Phys 70:634–644
Bellizzi AM, Bourne TD, Mills SE et al (2008) The cytologic features of sinonasal undifferentiated carcinoma and olfactory neuroblastoma. Am J Clin Pathol 129:367–376
Bogucki J, Taraszewska A, Czernicki Z (2004) Pure distant, leptomeningeal metastasis of esthesioneuro-epithelioma. Acta Neurochir (Wien) 146:1043–1045
Dahabreh I, Janinis D, Stamatelopoulos AG et al (2007) Surgical resection of esthesioneuroblastoma metastasis to the chest wall. J Thorac Oncol 2:93–95
Sharma S, Lasheen W, Walsh D (2008) Paraneoplastic refractory hypercalcemia due to advanced metastatic esthesioneuroblastoma. Rhinology 46:153–155
Wang XB, Pan XL, Wang TD (2005) Surgical approaches for sinonasal tumors with intracranial extension. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 40:363–365
Wick W, Wick A, Kuker W et al (2004) Intracranial metastatic esthesioneuroblastoma responsive to temozolomide. J Neuro-oncol 70:73–75
Theilgaard SA, Buchwald C, Ingeholm P et al (2003) Esthesioneuroblastoma: a Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol 123:433–439
Berger L, Luc R (1924) L'esthesioneuroepitheliome olfactif. Bull Assoc Fr Etude Cancer 13:410–421
Lee JY, Kim HK (2007) Primary olfactory neuroblastoma originating from the inferior meatus of the nasal cavity. Am J Otolaryngol 28:196–200
Broich G, Pagliari A, Ottaviani F (1997) Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924. Anticancer Res 17:2683–2706
Kleinschmidt-DeMasters BK, Pflaumer SM, Mulgrew TD et al (2000) Sinonasal teratocarcinosarcoma (“mixed olfactory neuroblastoma-craniopharyngioma”) presenting with syndrome of inappropriate secretion of antidiuretic hormone. Clin Neuropathol 19:63–69
Haas I, Ganzer U (2003) Does sophisticated diagnostic workup on neuroectodermal tumors have an impact on the treatment of esthesioneuroblastoma? Onkologie 26:261–267
Hwang SK, Paek SH, Kim DG et al (2002) Olfactory neuroblastomas: survival rate and prognostic factor. J Neuro-oncol 59:217–226
Ingeholm P, Theilgaard SA, Buchwald C et al (2002) Esthesioneuroblastoma: a Danish clinicopathological study of 40 consecutive cases. APMIS 110:639–645
Simon JH, Zhen W, McCulloch TM et al (2001) Esthesioneuroblastoma: the University of Iowa experience 1978–1998. Laryngoscope 111:488–493
Oskouian RJ Jr, Jane JA Sr, Dumont AS et al (2002) Esthesioneuroblastoma: clinical presentation, radiological, and pathological features, treatment, review of the literature, and the University of Virginia experience. Neurosurg Focus 12:e4
Nguyen BD, Roarke MC, Nelson KD et al (2006) F-18 FDG PET/CT staging and posttherapeutic assessment of esthesioneuroblastoma. Clin Nucl Med 31:172–174
Eriksen JG, Bastholt L, Krogdahl AS et al (2009) Esthesioneuroblastoma—what is the optimal treatment? Acta Oncol 39:231–235
Jethanamest D, Morris LG, Sikora AG et al (2007) Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg 133:276–280
Loy AH, Reibel JF, Read PW et al (2006) Esthesioneuroblastoma: continued follow-up of a single institution's experience. Arch Otolaryngol Head Neck Surg 132:134–138
Mishima Y, Nagasaki E, Terui Y et al (2004) Combination chemotherapy (cyclophosphamide, doxorubicin, and vincristine with continuous-infusion cisplatin and etoposide) and radiotherapy with stem cell support can be beneficial for adolescents and adults with estheisoneuroblastoma. Cancer 101:1437–1444
Rosenthal DI, Barker JL Jr, El-Naggar AK et al (2004) Sinonasal malignancies with neuroendocrine differentiation: patterns of failure according to histologic phenotype. Cancer 101:2567–2573
Kadish S, Goodman M, Wang CC (1976) Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 37:1571–1576
Gondim J, Ramos F Jr, Azevedo J et al (2002) Esthesioneuroblastoma: case report. Arq Neuropsiquiatr 60:303–307
Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RK (2004) Principles and Practice of Radiation Oncology, Lippincott Williams & Wilkins, 4th edn. (September 1, 2003), pp. 1132–3911
Diaz EM Jr, Johnigan RH 3rd, Pero C et al (2005) Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck 27:138–149
Zafereo ME, Fakhri S, Prayson R et al (2008) Esthesioneuroblastoma: 25-year experience at a single institution. Otolaryngol Head Neck Surg 138:452–458
Devaiah AK, Larsen C, Tawfik O et al (2003) Esthesioneuroblastoma: endoscopic nasal and anterior craniotomy resection. Laryngoscope 113:2086–2090
Castelnuovo PG, Delu G, Sberze F et al (2006) Esthesioneuroblastoma: endonasal endoscopic treatment. Skull Base 16:25–30
Bradley PJ, Jones NS, Robertson I (2003) Diagnosis and management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg 11:112–118
Resto VA, Eisele DW, Forastiere A et al (2000) Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck 22:550–558
Sheehan JM, Sheehan JP, Jane JA Sr et al (2000) Chemotherapy for esthesioneuroblastomas. Neurosurg Clin N Am 11:693–701
Dias FL, Sa GM, Lima RA et al (2003) Patterns of failure and outcome in esthesioneuroblastoma. Arch Otolaryngol Head Neck Surg 129:1186–1192
Constantinidis J, Steinhart H, Koch M et al (2004) Olfactory neuroblastoma: the University of Erlangen-Nuremberg experience 1975–2000. Otolaryngol Head Neck Surg 130:567–574
Rinaldo A, Ferlito A, Shaha AR et al (2002) Esthesioneuroblastoma and cervical lymph node metastases: clinical and therapeutic implications. Acta Otolaryngol 122:215–221
Lund VJ, Howard D, Wei W et al (2003) Olfactory neuroblastoma: past, present, and future? Laryngoscope 113:502–507
Liao H, Hua QQ, Wu ZY (2006) Maxillary swing approach in the management of tumors in the central and lateral cranial base. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 41:276–280
Josephs L, Jones L, Marenette L et al (2008) Cushing's syndrome: an unusual presentation of olfactory neuroblastoma. Skull Base 18:73–76
Yu J, Koch CA, Patsalides A et al (2004) Ectopic Cushing's syndrome caused by an esthesioneuroblastoma. Endocr Pract 10:119–124
Miura K, Mineta H, Yokota N et al (2001) Olfactory neuroblastoma with epithelial and endocrine differentiation transformed into ganglioneuroma after chemoradiotherapy. Pathol Int 51:942–947
Chacko G, Chandi SM, Chandy MJ (1998) Primary sphenoid and petrous apex esthesioneuroblastoma: a case report. Br J Neurosurg 12:264–266
Sarwar M (1979) Primary sellar–parasellar esthesioneuroblastoma. Am J Roentgenol 133:140–141
Mashberg A, Thoma KH, Wasilewski EJ (1979) Olfactory neuroblastoma (esthesioneuroblastoma) of the maxillary sinus. Oral Surg 13:908–912
Castro L, De La Pava S, Webster JH (1969) Esthesioneuroblastomas: a report of 7 cases. Am J Roentgenol Radium Ther Nucl Med 105:7–13
Levine PA, Gallagher R, Cantrell RW (1999) Esthesioneuroblastoma: reflections of a 21-year experience. Laryngoscope 109:1539–1543
Som PM, Lidov M, Brandwein M et al (1994) Sinonasal esthesioneuroblastoma with intracranial extension: marginal tumor cysts as a diagnostic MR finding. AJNR Am J Neuroradiol 15:1259–1262
Manelfe C, Bonafe A, Fabre P et al (1978) Computed tomography in olfactory neuroblastoma: one case of esthesioneuroepithelioma and four cases of esthesioneuroblastoma. J Comput Assist Tomogr 2:412–420
Yuan Y, Peng S, Xie Z (1999) Evaluation of CT in the diagnosis of esthesioneuroblastoma. Zhonghua Zhong Liu Za Zhi 21:134–135
Acknowledgement
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, T., Xu, YK., Li, L. et al. Esthesioneuroblastoma methods of intracranial extension: CT and MR imaging findings. Neuroradiology 51, 841–850 (2009). https://doi.org/10.1007/s00234-009-0581-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0581-0