Abstract
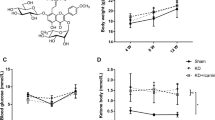
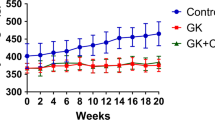
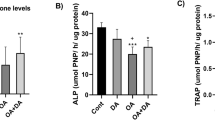
Metformin (Met), an anti-diabetes drug, has also shown therapeutic effects for ovariectomy-induced (OVX) osteoporosis. However, similar effects against bone loss induced by a ketogenic diet (KD) have not been tested. This study was aimed to evaluate the microarchitectures and biomechanics of KD-induced osteoporosis with and without administration of Met, and compare the effect of Met on bone loss induced by KD with OVX. Forty female C57BL/6J mice were randomly divided into Sham, OVX, OVX + Met (100 mg/kg/day), KD (3:1 ratio of fat to carbohydrate and protein), and KD + Met (100 mg/kg/day) groups. After 12 weeks, the bone mass and biomechanics were measured in distal cancellous bone and in mid-shaft cortical bone of the femur. The activities of serum alkaline phosphatase (ALP) and tartrate-resistant acid phosphatase (TRAP), together with immunohistochemistry staining of osteocalcin (OCN) and TRAP, were evaluated. Both OVX and KD induced significant bone loss and compromised biomechanical properties in the cancellous bone, but no effect was found in cortical bone. The administration of Met increased the cancellous bone volume fraction (BV/TV) from 3.78 to 5.23% following the OVX and from 4.04 to 6.33% following the KD, it also enhanced the compressive stiffness from 47 to 160 N/mm following the OVX and from 35 to 340 N/mm with the KD. Met effectively increased serum ALP in the KD group while decreased serum TRAP in the OVX group, but up-regulated expression of OCN and down-regulated expression of TRAP in both OVX and KD groups. The present study demonstrated that Met effectively attenuated the cancellous bone loss induced by KD and maintained the biomechanical properties of long bones, providing evidence for Met as a treatment of by KD-induced osteoporosis in teenage skeleton.





Similar content being viewed by others
References
Kossoff EH, Zupec-Kania BA, Rho JM (2009) Ketogenic diets: an update for child neurologists. J Child Neurol 24:979–988
Stafstrom CE, Rho JM (2012) The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 3:59
Paoli A, Rubini A, Volek JS, Grimaldi KA (2013) Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 67:789–796
Wang X, Wu X, Liu Q, Kong G, Zhou J, Jiang J, Wu X, Huang Z, Su W, Zhu Q (2017) Ketogenic metabolism inhibits histone deacetylase (HDAC) and reduces oxidative stress after spinal cord injury in rats. Neuroscience 366:36–43
Kong G, Huang Z, Ji W, Wang X, Liu J, Wu X, Huang Z, Li R, Zhu Q (2017) The ketone metabolite beta-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J Neurotrauma 34:2645–2655
Hahn TJ, Halstead LR, DeVivo DC (1979) Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int 28:17–22
Bergqvist AG, Schall JI, Stallings VA, Zemel BS (2008) Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr 88:1678–1684
Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, Bidlingmaier M (2010) Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res 25:275–284
Wu X, Huang Z, Wang X, Fu Z, Liu J, Huang Z, Kong G, Xu X, Ding J, Zhu Q (2017) Ketogenic diet compromises both cancellous and cortical bone mass in mice. Calcif Tissue Int 101:412–421
Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S (2016) Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 164:740–751
Vestergaard P, Rejnmark L, Mosekilde L (2005) Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48:1292–1299
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T (2008) Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun 375:414–419
Cortizo AM, Sedlinsky C, McCarthy AD, Blanco A, Schurman L (2006) Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol 536:38–46
Zhen D, Chen Y, Tang X (2010) Metformin reverses the deleterious effects of high glucose on osteoblast function. J Diabetes Complications 24:334–344
Gao Y, Li Y, Xue J, Jia Y, Hu J (2010) Effect of the anti-diabetic drug metformin on bone mass in ovariectomized rats. Eur J Pharmacol 635:231–236
Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP, Zhao L, Jia CH, Wen ZH, Jin DD, Bai XC (2011) Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem 112:2902–2909
Reeves PG (1997) Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 127:838S–841S
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Arias-Moreno AJ, Ito K, van Rietbergen B (2016) Micro-Finite Element analysis will overestimate the compressive stiffness of fractured cancellous bone. J Biomech 49:2613–2618
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191
Qi S, Zheng H (2017) Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res 177:281–287
Unsal F, Sonmez MF (2014) The effects of ovariectomy on ghrelin expression in the rat uterus. Adv Clin Exp Med 23:363–370
Li M, Shen Y, Wronski TJ (1997) Time course of femoral neck osteopenia in ovariectomized rats. Bone 20:55–61
Danielsen CC, Mosekilde L, Svenstrup B (1993) Cortical bone mass, composition, and mechanical properties in female rats in relation to age, long-term ovariectomy, and estrogen substitution. Calcif Tissue Int 52:26–33
Sasaki H, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Kamo K, Shimada Y (2010) Effects of combination treatment with alendronate and vitamin K(2) on bone mineral density and strength in ovariectomized mice. J Bone Miner Metab 28:403–409
Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RJ, de Cabo R, Ulrich S, Akassoglou K, Verdin E (2013) Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339:211–214
Wang X, Liu Q, Zhou J, Wu X, Zhu Q (2017) β Hydroxybutyrate levels in serum and cerebrospinal fluid under ketone body metabolism in rats. Exp Anim 66:177–182
Frommelt L, Bielohuby M, Stoehr BJ, Menhofer D, Bidlingmaier M, Kienzle E (2014) Effects of low-carbohydrate, high-fat diets on apparent digestibility of minerals and trace elements in rats. Nutrition 30:869–875
Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P (2013) Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med 16:277–286
Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D (2013) Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153:228–239
Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ (2010) Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 33:322–326
Chandra A, Lin T, Tribble MB, Zhu J, Altman AR, Tseng WJ, Zhang Y, Akintoye SO, Cengel K, Liu XS, Qin L (2014) PTH1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone 67:33–40
Raisz LG (1999) Physiology and pathophysiology of bone remodeling. Clin Chem 45:1353–1358
Bahlous A, Kalai E, Hadj SM, Bouzid K, Zerelli L (2006) Biochemical markers of bone remodeling: recent data of their applications in managing postmenopausal osteoporosis. Tunis Med 84:751–757
Hwang YH, Son YJ, Paik MJ, Yee ST (2017) Effects of diisononyl phthalate on osteopenia in intact mice. Toxicol Appl Pharmacol 334:120–128
Greenblatt MB, Tsai JN, Wein MN (2017) Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem 63:464–474
Lin S, Huang J, Fu Z, Liang Y, Wu H, Xu L, Sun Y, Lee WY, Wu T, Qin L, Cui L, Li G (2015) The effects of atorvastatin on the prevention of osteoporosis and dyslipidemia in the high-fat-fed ovariectomized rats. Calcif Tissue Int 96:541–551
Zhao Y, Zou B, Shi Z, Wu Q, Chen GQ (2007) The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials 28:3063–3073
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608
Komori T (2015) Animal models for osteoporosis. Eur J Pharmacol 759:287–294
Comelekoglu U, Mutlu H, Yalin S, Bagis S, Yildiz A, Ogenler O (2007) Determining the biomechanical quality of normal and osteoporotic bones in rat femora through biomechanical test and finite element analysis. Acta Orthop Traumatol Turc 41:53–57
van Eijden TM, van Ruijven LJ, Giesen EB (2004) Bone tissue stiffness in the mandibular condyle is dependent on the direction and density of the cancellous structure. Calcif Tissue Int 75:502–508
Acknowledgements
This study was supported by Guangdong Province Natural Science Foundation of China (No. 2015A030313276) and Dean Foundation of Nanfang Hospital (No. 2016Z021).
Author information
Authors and Affiliations
Contributions
QZ and QL designed the experiments. QL, ZY, and XX conducted the animal experiments. QL, ZY, JL, and YL collected the samples. QL, YZ, XX, XW and ZH measured and collected the data. ZH, GK, JD, RL and JL completed the data analysis. QL wrote the manuscript, and XX and QZ revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Qi Liu, Xiaolin Xu, Zhou Yang, Yapu Liu, Xiuhua Wu, Zhiping Huang, Junhao Liu, Zucheng Huang, Ganggang Kong, Jianyang Ding, Rong Li, Junyu Lin, and Qingan Zhu declare no conflicts of interest.
Human and Animal Rights and Informed Consent
The present study was approved by the Animal Experiments Ethics Committee of Southern Medical University. The animal procedures were conducted in accordance with the Guidelines of Caring for Laboratory Animals by the Ministry of Science and Technology of the People’s Republic of China. Surgery was performed under anesthesia, and all efforts were made to minimize suffering.
Rights and permissions
About this article
Cite this article
Liu, Q., Xu, X., Yang, Z. et al. Metformin Alleviates the Bone Loss Induced by Ketogenic Diet: An In Vivo Study in Mice. Calcif Tissue Int 104, 59–69 (2019). https://doi.org/10.1007/s00223-018-0468-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0468-3