Abstract
The study aimed to investigate the volume of the olfactory bulb in smokers. Specifically, we wanted to see whether environmental influences may exert a negative influence on OB structure. Twenty-one smokers and 59 non-smokers, matched for age and sex, underwent olfactory testing by means of the Sniffin’ Sticks testing device (measurement of odor threshold and identification abilities). In addition, they underwent an MR scan with 2-mm-thick T2-weighted fast spin-echo images without interslice gap in the coronal plane covering the anterior and middle segments of the base of the skull. Olfactory function was not different between the 2 groups; however, olfactory bulb volumes were smaller in smokers than in non-smokers (p = 0.006). The deficit seen at the level of the OB did not correlate with the duration of smoking. The current data indicate that smoking may have a negative effect on the olfactory system before this becomes obvious in terms of a decreased olfactory function.
Similar content being viewed by others
Introduction
The olfactory bulb (OB) has become of increasing scientific interest in the last years for many reasons, for example, at least in animals, it is one of few areas in the brain, where adult neurogenesis continuously takes place (Lledo and Gheusi 2003; Curtis et al. 2007; Whitman and Greer 2009; Ming and Song 2011); cells migrate from the subventricular zone via the rostral migratory stream into the OB and differentiate into interneurons (Whitman and Greer 2009; Ming and Song 2011). In addition, the first synapse of the olfactory receptor neurons to the mitral cells is located in the OB (Lledo and Gheusi 2003). Here, the information is modulated, integrated and transmitted to higher brain areas.
One method to examine the OB, especially applicable in humans, is the measurement of the OB volume (Yousem et al. 1997; Rombaux et al. 2009) using magnetic resonance imaging (MRI). Results have shown that the OB volume changes with age (Buschhuter et al. 2008; Hummel et al. 2011). In children and adolescents, the OB volume increases (Hummel et al. 2011) and decreases again with aging (Buschhuter et al. 2008). Previous studies reported a close connection between the OB volume and olfactory function (Buschhuter et al. 2008; Haehner et al. 2008). Thus, the volume of the OB is positively correlated with olfactory performance. In addition, the volume of the OB has shown to be affected by diseases related to olfactory dysfunction. The range of disease is wide, from chronic rhinosinusitis (Gudziol et al. 2009) over post-traumatic olfactory dysfunction (Jiang et al. 2009) over Parkinson’s disease (Brodoehl et al. 2012) to schizophrenia (Turetsky et al. 2000) and others (Thomann et al. 2009; Goektas et al. 2011; Podlesek et al. 2012). In all of the above mentioned studies, the volume of the OB was decreased compared to healthy controls. Most publications explain the changed OB volume with an insufficient afferent input of olfactory information from the olfactory receptor neurons to the OB (Gudziol et al. 2009).
The plasticity of the OB was observed in animal models by reducing the input of olfactory information by naris closure or ablation of the olfactory epithelium (Caggiano and Brunjes 1993; Cummings et al. 1997; Paskin et al. 2011). Here, the volume of the OB was significant reduced and recovered after reopening of the naris (Cummings et al. 1997). In line with these results, plasticity of the OB was reported in humans (Haehner et al. 2008; Gudziol et al. 2009). Gudziol and colleagues were able to show an increase in OB volume after successful treatment of patients with chronic rhinosinusitis (Gudziol et al. 2009). Another study by Haehner et al. observed changes of OB volume in relation to the changes of olfactory function. A strong correlation between changes of volume and changes of olfactory function had been found (Haehner et al. 2008).
The role of smoking on olfaction is discussed controversially. Animal studies have shown that smoking not only has an effect on the sense of smell, but also on cell differentiation in the OB (Chen et al. 1999). To our knowledge, this topic has not been addressed in humans. Therefore, the aim of the current study was to examine the OB volume by means of MR imaging of smokers compared to non-smokers.
Materials and methods
Participants
Participants included 21 smokers (9 men, 12 women; mean age, 22.5 years; age range, 19–32 years; all smokers currently smoked more than 3 cigarettes per day) and 59 non-smoking control subjects (23 men, 26 women; mean age, 23.9 years; age range, 19–28 years) matched for gender and age (meaning there was no significant difference in age [t = 1.71, p = 0.099] or in gender distribution between groups [χ2 = 1.06, p = 0.22]). Non-smoking controls were taken from a previous investigation (Buschhuter et al. 2008) that utilized exactly the same techniques for investigating the OB and olfactory function. In fact, from this previous study, all subjects between an age of 19 and 28 were included.
Detailed information about the experiment was given to all participants and written consent was obtained. All aspects of the study were performed in accordance with the Declaration of Helsinki. The study protocol was approved by the local Ethics Board of the Faculty of Medicine of the Technical University of Dresden.
Olfactory testing
In all subjects, a detailed history was taken. They were instructed not to eat or to drink anything but water 1 hour prior to the measurements, in order to avoid chemosensory desensitization. Odor thresholds for phenylethylalcohol (a rose-like odor) were assessed monorhinally in all individuals by means of the “Sniffin’ Sticks” test kit (Hummel et al. 1997; Kobal et al. 2000). In addition, odor identification was measured in a birhinal fashion using the 16-item kit from the “Sniffin’ Sticks”.
Magnetic resonance imaging (MRI)
All examinations were performed at a 1.5-Tesla magnetic resonance imaging system (Sonata Vision; Siemens, Erlangen, Germany) using the cp-head coil. Volumes of the right and left OB were determined using a 2-mm-thick T2-weighted fast spin-echo images without interslice gap in the coronal plane covering the anterior and middle segments of the base of the skull. Measurements of OB volume were performed by the manual segmentation of the coronal slices through the OBs using the AMIRA 3D visualization and modeling system (Visage Imaging, Carlsbad, USA). OB volumes were calculated by planimetric manual contouring (surface in mm2), and all surfaces were added and multiplied by 2 because of the 2 mm slice thickness to obtain a volume in mm3. The volumetric measurement has previously been described in great detail (Rombaux et al. 2009). In current study, we used the same anatomical landmarks. As the anterior boarder, the MR slice where the OB becomes visible was used. The posterior boarder of the OB was set to the MR slice where the OB changes from a round to an oval shape/where a steep decrease in diameter is visible. This change marks the transition from the OB to the olfactory tract.
Statistical analyses
Data were analyzed by means of SPSS 19.0 (SPSS Inc., Chicago, IL, USA). T tests were used wherever appropriate. The level of significance was set at 0.05.
Results
In total, 80 participants were included in the study (21 smokers, 59 non-smokers). The olfactory function (odor threshold and odor identification) was measured by means of the “Sniffin’ Sticks”, and OB volumes were obtained by MR imaging. The results are divided into two major parts—psychophysical function (olfaction) and anatomy (OB volumes). Table 1 summarizes the results.
Psychophysical function: smokers versus non-smokers
Olfactory function was assessed using the “Sniffin’ Sticks”. When comparing birhinal measurements for odor identification, non-smokers (M = 14.0, SD = 1.2) scored within the same range as smokers (M = 14.2, SD = 1.0) (t test: t [78] = 0.86, p = 0.39). Also, the odor threshold test did not reveal a significant difference between non-smokers and smokers (non-smokers: M = 7.93, SD = 2.12; smokers: M = 6.94, SD = 2.58) (t test: t [78] = 1.73, p = 0.09).
Anatomy OB volume: smokers versus non-smokers
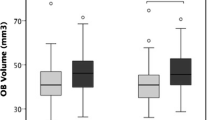
Anatomical MR images were obtained to measure the OB volume of all subjects. When comparing OB volumes between the two groups, smokers (left M = 59.9 mm3, SD = 17.7; right M = 59.5 mm3, SD = 15.6) had significantly smaller OB volumes than non-smokers (left M = 71.6 mm3, SD = 13.6; right M = 72.3 mm3, SD = 12.3) for the left as well as for the right OB as indicated by an analysis of variance for repeated measures (factor “smoker” (F [1,78] = 6.98, p = 0.01; factor “side” (F [1,78] = 0.23, p = 0.63; interaction between factors “smoker” × “side” (F [1,78] = 1.22, p = 0.27)) (Fig. 1).
Correlation between duration of smoking an OB volume
The average duration of smoking was 7.5 years (SD = 3.8; range 2–14 years). When correlating duration of smoking with olfactory function and OB volume (controlling for age in a partial correlation design), no significant correlation emerged (r 18 < 0.40, p > 0.08).
Discussion
In this study, we observed a significantly reduced OB volume in smokers compared to non-smokers. No significant difference in odor identification and odor threshold performance was found between the two groups.
Several studies have addressed the impact of smoking on the sense of smell. The data reported so far are contradictory. Some studies found reduced olfactory function in smokers (Frye et al. 1990; Ishimaru and Fujii 2007; Katotomichelakis et al. 2007; Vennemann et al. 2008). In particular, olfactory threshold was reduced which is associated with peripheral olfactory function (Katotomichelakis et al. 2007). Other studies reported a selective olfactory deficit to odors included in cigarette smoke (Ahlstrom et al. 1987; Rosenblatt et al. 1998). In contrast, some authors did not observe an effect of smoking on the sense of smell (Venstrom and Amoore 1968; McLean et al. 2004; Yee et al. 2009; Orhan et al. 2011). In our study, olfactory performance of smokers and non-smokers was within the same range. The average duration of smoking was 7.5 years (SD 3.8). Frye et al. showed a negative correlation between cumulative smoking dosage and odor identification ability (Frye et al. 1990). So, the data on olfactory function in smokers are not totally homogeneous. Our psychophysical data seem to add to the standpoint that smoking has no major effect yet on odor identification and odor threshold performance, at least in smokers with an average smoking duration of 7.5 years or less, possibly owing to the plasticity of the olfactory system. The non-significant difference between smokers and non-smokers (p = 0.09), however, may also represent a type-2 error, which may be due the relatively small sample size.
Changes in OB volume are believed to be causes by insufficient input from the olfactory receptor neurons due to a variety of reasons—a “bottom-up mechanism” is discussed. This was demonstrated in experiments based on naris closure and olfactory epithelium ablation. Here, the afferent input to the olfactory bulb was minimized, which resulted in a reduced OB volume (Caggiano and Brunjes 1993; Cummings et al. 1997; Paskin et al. 2011). This effect was reversible after reopening of the nostril (Cummings et al. 1997). In humans, surgical treatment of patients with chronic rhinosinusitis resulted in an increase of OB volume (Gudziol et al. 2009). The authors explain this volume change with an increase in olfactory function and therefore a restored afferent input to the OB.
The olfactory epithelium of smokers has been histologically examined, and the results show a squamous metaplasia and changed morphology of olfactory receptor neurons in smokers (Yee et al. 2009). Vent et al. used a rat model to observe the effect of tobacco smoke on the olfactory receptor neurons. Apoptosis of olfactory receptor neurons was significantly increased in these animals compared to a control group (Vent et al. 2004). These findings suggest a “bottom-up mechanism” as the reason for OB reduction in smokers, like it is described as the mechanism of reduced OB volumes in patients with olfactory loss (Gudziol et al. 2009).
Another approach to explain our findings could be a direct effect of nicotine on the neurogenesis/synaptogenesis of the OB. New cells migrate from the subventricular zone via the rostral migratory stream to the OB and differentiate (Curtis et al. 2007; Whitman and Greer 2009). It has been shown that nicotine reduces neurogenesis and promotes glia genesis (Bruijnzeel et al. 2011). In another study, rats were given nicotine orally and similar results were obtained—mitral cells in the OB were reduced by 32 % (Chen et al. 1999). These results show a direct effect of nicotine on the OB. In particular, the study by Chen et al. in which nicotine was applied orally supports a direct influence of nicotine on the volume of the OB independent of the reduced afferent input to the OB.
To our knowledge, this is the first study to show in human that the OB volume is reduced in smokers. Interestingly, in our study, smokers performed as well as non-smokers on the olfactory tests. It can be concluded that reduction of OB volume occurs early as a negative effect of smoking on the olfactory system even before this damage of the sense of smell becomes visible in standard olfactory tests. The mechanism, which results in this reduction of OB volume in smokers, remains unclear. Considering results from previous studies, a dual mechanism with direct action of nicotine on the neurogenesis/synaptogenesis of the OB and an insufficient afferent input due to changes in the olfactory epithelium caused by tobacco smoke seems plausible to explain our findings.
As mentioned above, the control group was taken from a previous published study (Buschhuter et al. 2008). The OB volumes of the two groups were measured by different raters. This could be a possible limitation of the study. All OB volumes of the 21 smokers (in total 41 OB) were retraced by a third rater. The difference between the results was marginal with a mean of 3.11 % (SD 2.6). Analysis of inter-rater reliability by means of the intraclass correlation coefficient (ICC) revealed a Cronbach’s Alpha of 0.993 which indicates a high concordance between the two raters. Therefore, we presume that the difference between the control group and the group of smokers in OB volume is not due to different raters but is related to the factor “smoking”.
Results from the current study may affect the interpretation of future studies on the volume of the olfactory bulb, for example, in patients with schizophrenia who often are smokers (e.g., Winterer 2010; Turetsky et al. 2000). In other words, future studies on OB volume should consider smoking behavior.
In conclusion, the current data indicate that smoking may have a negative effect on the olfactory system before this becomes obvious in terms of a decreased olfactory function. Further studies are needed to address the question whether the reduced OB volume is reversible when smokers quit smoking.
References
Ahlstrom R, Berglund B, Berglund U, Engen T, Lindvall T (1987) A comparison of odor perception in smokers, nonsmokers, and passive smokers. Am J Otolaryngol 8:1–6
Brodoehl S, Klingner C, Volk GF, Bitter T, Witte OW, Redecker C (2012) Decreased olfactory bulb volume in idiopathic Parkinson’s disease detected by 3.0-Tesla magnetic resonance imaging. Mov Disord 27:1019–1025
Bruijnzeel AW, Bauzo RM, Munikoti V et al (2011) Tobacco smoke diminishes neurogenesis and promotes gliogenesis in the dentate gyrus of adolescent rats. Brain Res 1413:32–42. doi:10.1016/j.brainres.2011.07.041
Buschhuter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND, Hummel T (2008) Correlation between olfactory bulb volume and olfactory function. Neuroimage 42:498–502
Caggiano AO, Brunjes PC (1993) Microglia and the developing olfactory bulb. Neuroscience 52:717–724
Chen WJ, Parnell SE, West JR (1999) Effects of alcohol and nicotine on developing olfactory bulb: loss of mitral cells and alterations in neurotransmitter levels. Alcohol Clin Exp Res 23:18–25
Cummings DM, Henning HE, Brunjes PC (1997) Olfactory bulb recovery after early sensory deprivation. J Neurosci 17:7433–7440
Curtis MA, Kam M, Nannmark U et al (2007) Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315:1243–1249
Frye RE, Schwartz BS, Doty RL (1990) Dose-related effects of cigarette smoking on olfactory function. JAMA 263:1233–1236
Goektas O, Schmidt F, Bohner G et al (2011) Olfactory bulb volume and olfactory function in patients with multiple sclerosis. Rhinology 49:221–226
Gudziol V, Buschhuter D, Abolmaali N, Gerber J, Rombaux P, Hummel T (2009) Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—a longitudinal study. Brain 132:3096–3101
Haehner A, Rodewald A, Gerber JC, Hummel T (2008) Correlation of olfactory function with changes in the volume of the human olfactory bulb. Arch Otolaryngol Head Neck Surg 134:621–624
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52
Hummel T, Smitka M, Puschmann S, Gerber JC, Schaal B, Buschhuter D (2011) Correlation between olfactory bulb volume and olfactory function in children and adolescents. Exp Brain Res 214:285–291
Ishimaru T, Fujii M (2007) Effects of smoking on odour identification in Japanese subjects. Rhinology 45:224–228
Jiang RS, Chai JW, Chen WH, Fuh WB, Chiang CM, Chen CC (2009) Olfactory bulb volume in taiwanese patients with posttraumatic anosmia. Am J Rhinol Allergy 23:582–584
Katotomichelakis M, Balatsouras D, Tripsianis G, Davris S, Maroudias N, Danielides V, Simopoulos C (2007) The effect of smoking on the olfactory function. Rhinology 45:273–280
Kobal G, Klimek L, Wolfensberger M et al (2000) Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur arch otorhinolaryngol 257:205–211
Lledo PM, Gheusi G (2003) Olfactory processing in a changing brain. Neuroreport 14:1655–1663
McLean D, Feron F, Mackay-Sim A, McCurdy R, Hirning M, Chant D, McGrath J (2004) Paradoxical association between smoking and olfactory identification in psychosis versus controls. Aust N Z J psychiatry 38:81–83
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702
Orhan KS, Karabulut B, Keles N, Deger K (2011) Evaluation of factors concerning the olfaction using the sniffin’ sticks test. Otolaryngol Head Neck Surg. doi:10.1177/0194599811425019
Paskin TR, Iqbal TR, Byrd-Jacobs CA (2011) Olfactory bulb recovery following reversible deafferentation with repeated detergent application in the adult zebrafish. Neuroscience 196:276–284
Podlesek D, Leimert M, Schuster B, Gerber J, Schackert G, Kirsch M, Hummel T (2012) Olfactory bulb volume in patients with idiopathic normal pressure hydrocephalus. Neuroradiology doi. doi:10.1007/s00234-012-1050-8
Rombaux P, Grandin C, Duprez T (2009) How to measure olfactory bulb volume and olfactory sulcus depth? B-ENT 5(Suppl 13):53–60
Rosenblatt MR, Olmstead RE, Iwamoto-Schaap PN, Jarvik ME (1998) Olfactory thresholds for nicotine and menthol in smokers (abstinent and nonabstinent) and nonsmokers. Physiol Behav 65:575–579
Thomann PA, Dos Santos V, Toro P, Schonknecht P, Essig M, Schroder J (2009) Reduced olfactory bulb and tract volume in early Alzheimer’s disease—a MRI study. Neurobiol Aging 30:838–841
Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE (2000) Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry 157:828–830
Vennemann MM, Hummel T, Berger K (2008) The association between smoking and smell and taste impairment in the general population. J Neurol 255:1121–1126
Venstrom D, Amoore J (1968) Olfactory threshold in relation to age, sex or smoking. J Food Sci 33:264–265
Vent J, Robinson AM, Gentry-Nielsen MJ, Conley DB, Hallworth R, Leopold DA, Kern RC (2004) Pathology of the olfactory epithelium: smoking and ethanol exposure. Laryngoscope 114:1383–1388
Whitman MC, Greer CA (2009) Adult neurogenesis and the olfactory system. Prog Neurobiol 89:162–175
Winterer G (2010) Why do patients with schizophrenia smoke? Curr Opin Psychiatry 23:112–119
Yee KK, Pribitkin EA, Cowart BJ et al (2009) Smoking-associated squamous metaplasia in olfactory mucosa of patients with chronic rhinosinusitis. Toxicol Pathol 37:594–598
Yousem DM, Geckle RJ, Doty RL, Bilker WB (1997) Reproducibility and reliability of volumetric measurements of olfactory eloquent structures. Acad Radiol 4:264–269
Acknowledgments
We are thankful to Dorothee Buschhüter as she provided data from healthy subject; we also would like to thank José R.B. Galván, Vasyl Bogdanov, and Marta Leal Bento for their help in data analysis. This research was supported by the Roland-Ernst-Stiftung.
Conflict of interest
None of the authors has any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schriever, V.A., Reither, N., Gerber, J. et al. Olfactory bulb volume in smokers. Exp Brain Res 225, 153–157 (2013). https://doi.org/10.1007/s00221-012-3356-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3356-5