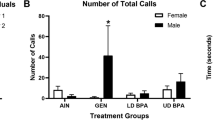
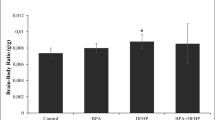
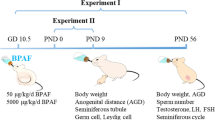
Abstract
Two potential endocrine-disrupting chemicals, bisphenol A (BPA) and nonylphenol (NP), were assessed for their long-lasting effects on endocrine/reproductive systems following transplacental and lactational exposure to rat offspring during a time-window that included the critical period for brain sexual differentiation. Each chemical was mixed with diet at concentrations of 60, 600 and 3000 ppm and was provided to maternal Sprague-Dawley rats from gestational day (GD) 15 to postnatal day (PND) 10. Ethinylestradiol (EE) at 0.5 ppm was used as an estrogenic reference drug. During pregnancy and lactation, including the exposure period, a soy-free rodent diet was provided to eliminate possible modification of the study results by plant-derived phytoestrogens. Effects on endocrine/reproductive systems were evaluated by examining the anogenital distance, organ weights before puberty, onset of puberty, estrous cyclicity, and organ weights and histopathology of adult endocrine organs (at 11 weeks of age), as well as the volume of the sexually dimorphic nucleus of preoptic area. Both NP and BPA, at high doses, caused decreases in maternal body weights and retardation of offspring growth, but neither affected any of the endocrine/reproductive endpoints of offspring, whereas EE induced irreversible changes in estrous cyclicity and histopathology of ovaries and uterus of adult females. The results indicated that maternal dietary exposure to NP or BPA at concentrations up to 3000 ppm from GD 15 through PND 10 do not exert any apparent adverse effects on the endocrine/reproductive systems of offspring.

Similar content being viewed by others
References
Ashby J (2001) Testing for endocrine disruption post-EDSTAC: extrapolation of low dose rodent effects to humans. Toxicol Lett 120:233–242
Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM (1999) Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology 140:5364–5373
Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM (2000) Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology 141:3898–3907
Bjerke DL, Brown TJ, MacLusky NJ, Hochberg RB, Peterson RE (1994) Partial demasculinization and feminization of sex behavior in male rats by in utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin is not associated with alterations in estrogen receptor binding or volumes of sexually differentiated brain nuclei. Toxicol Appl Pharmacol 127:258–267
Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Makela SA (1998) A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environ Health Perspect 106:369–373
Chahoud I, Fialkowski O, Talsness CE (2001) The effects of low and high dose in utero exposure to bisphenol A on the reproductive system of male rat offspring. Reprod Toxicol 15:589
Chapin RE, Delaney J, Wang Y, Lanning L, Davis B, Collins B, Mintz N, Wolfe G (1999) The effects of 4-nonylphenol in rats: a multigeneration reproduction study. Toxicol Sci 52:80–91
Davies MJ, Norman RJ (2002) Programming and reproductive functioning. Trends Endocrinol Metab 13:386–392
Davis EC, Shryne JE, Gorski RA (1995) A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinology 62:579–585
Doerge DR, Twaddle NC, Churchwell MI, Chang HC, Newbold RR, Delclos KB (2002) Mass spectrometric determination of p-nonylphenol metabolism and disposition following oral administration to Sprague-Dawley rats. Reprod Toxicol 16:45–56
Faber KA, Hughes CL Jr (1993) Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotrophin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod Toxicol 7:35–39
Ferguson SA, Scallet AC, Flynn KM, Meredith JM, Schwetz BA (2000) Developmental neurotoxicity of endocrine disrupters: focus on estrogens. Neurotoxicology 21:947–956
Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G (1999) Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol 158:231–243
Hopert AC, Beyer A, Frank K, Strunck E, Wunsche W, Vollmer G (1998) Characterization of estrogenicity of phytoestrogens in an endometrial-derived experimental model. Environ Health Perspect 106:581–586
Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK (1994) SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav 56:535–541
Kurebayashi H, Betsui H, Ohno Y (2003) Disposition of a low dose of 14C-bisphenol A in male rats and its main biliary excretion as BPA glucuronide. Toxicol Sci 73:17–25
Kwon S, Stedman DB, Elswick BA, Cattley RC, Welsch F (2000) Pubertal development and reproductive functions of Crl:CD BR Sprague-Dawley rats exposed to bisphenol A during prenatal and postnatal development. Toxicol Sci 55:399–406
Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M (2003) Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology (in press) DOI 10.1016/S0300-483X(03)00269-5
Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Gallo M, Reuhl K, Ho SM, Brown T, Moore J, Leakey J, Haseman J, Kohn M (2002) Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review. Environ Health Perspect 110:427–431
Meredith JM, Bennett C, Scallet AC (2001) A practical three-dimensional reconstruction method to measure the volume of the sexually-dimorphic central nucleus of the medial preoptic area (MPOC) of the rat hypothalamus. J Neurosci Methods 104:113–121
Morrissey RE, George JD, Price CJ, Tyl RW, Marr MC, Kimmel CA (1987) The developmental toxicity of bisphenol A in rats and mice. Fundam Appl Toxicol 8:571–582
Nagao T, Saito Y, Usumi K, Kuwagata M, Imai K (1999) Reproductive function in rats exposed neonatally to bisphenol A and estradiol benzoate. Reprod Toxicol 13:303–311
Nagao T, Saito Y, Usumi K, Nakagomi M, Yoshimura S, Ono H (2000) Disruption of the reproductive system and reproductive performance by administration of nonylphenol to newborn rats. Hum Exp Toxicol 19:284–296
Nagao T, Wada K, Marumo H, Yoshimura S, Ono H (2001) Reproductive effects of nonylphenol in rats after gavage administration: a two-generation study. Reprod Toxicol 15:293–315
Newbold RR (1999) Hormonal mechanisms in female reproductive tract toxicity. In: Harvey PW, Rush KC and Cockburn A (eds) Endocrine and hormonal toxicology. John Wiley, Chichester, pp 406–417
Noto T, Oishi Y, Fujihira S, Matsumoto M, Yoshizawa K, Tsubota K, Hashimoto M, Ohara K (1999) Spontaneous lesions in Crj:CD(SD)IGS rats versus in Jcl:SD rats used in toxicity studies—histopathological findings. In: Matsuzawa T, Inoue H (eds) Biological reference data on CD(SD)IGS rats—1999. Best Printing, Tokyo, pp 23–28
Odum J, Ashby J (2000) Neonatal exposure of male rats to nonylphenol has no effect on the reproductive tract. Toxicol Sci 56:400–404
Odum J, Lefevre PA, Tinwell H, Van Miller JP, Joiner RL, Chapin RE, Wallis NT, Ashby J (2002) Comparison of the developmental and reproductive toxicity of diethylstilbestrol administered to rats in utero, lactationally, preweaning, or postweaning. Toxicol Sci 68:147–163
Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM Jr (2000) The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci 54:3–18
Register B, Bethel MA, Thompson N, Walmer D, Blohm P, Ayyash L, Hughes C Jr (1995) The effect of neonatal exposure to diethylstilbestrol, coumestrol, and β-sitosterol on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Proc Soc Exp Biol Med 208:72–77
Rhees RW, Shryne JE, Gorski RA (1990a) Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol 21:781–786
Rhees RW, Shryne JE, Gorski RA (1990b) Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res 52:17–23
Rubin BS, Murray MK, Damassa DA, King JC, Soto AM (2001) Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 109:675–680
Shibutani M, Uneyama C, Masutomi N, Abe N, Takahashi N, Nakagawa K, Hirose M (2001) Dose-response study of perinatally exposed ethinylestradiol on the expression of ERE-containing genes in the SDN-POA of neonatal rats. Toxicologist 60 [Suppl 1]:295
Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SCJ, Fennell TR (2000) Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 168:225–234
Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T (2002) Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol 16:107–116
Takahashi O, Oishi S (2000) Disposition of orally administered 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect 108:931–935
Talsness CE, Wu X, Witthoft W, Chahoud I (2001) The effects of low and high dose in utero exposure to bisphenol A on the reproductive system of female rat offspring. Reprod Toxicol 15:596
Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe B (1999) Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci 49:530–536
Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J (2002) Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci 68:339–348
Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM (2002) Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci 68:121–146
Acknowledgements
This work was supported in part by Health and Labour Sciences Research Grants (Research on Food and Chemical Safety) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takagi, H., Shibutani, M., Masutomi, N. et al. Lack of maternal dietary exposure effects of bisphenol A and nonylphenol during the critical period for brain sexual differentiation on the reproductive/endocrine systems in later life. Arch Toxicol 78, 97–105 (2004). https://doi.org/10.1007/s00204-003-0517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0517-0