Abstract
Summary
In a 5-year study involving 119 postmenopausal women, zoledronic acid 4 mg given once-yearly for 2, 3 or 5 years was well tolerated with no evidence of excessive bone turnover reduction or any safety signals. BMD increased significantly. Bone turnover markers decreased from baseline and were maintained within premenopausal reference ranges.
Introduction
After completion of the core study, two consecutive, 2-year, open-label extensions investigated the efficacy and safety of zoledronic acid 4 mg over 5 years in postmenopausal osteoporosis.
Methods
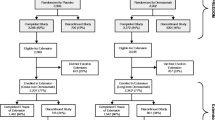
In the core study, patients received 1 to 4 mg zoledronic acid or placebo. In the first extension, most patients received 4 mg per year and then patients entered the second extension and received 4 mg per year or calcium only. Patients were divided into three subgroups according to years of active treatment received (2, 3 or 5 years). Changes in BMD and bone turnover markers (bone ALP and CTX-I) were assessed.
Results
All subgroups showed substantial increases in BMD and decreases in bone markers. By the end of the core study, 37.5% of patients revealed a suboptimal reduction (< 30%) of bone ALP levels. After subsequent study drug administration during the extensions, there was no evidence of progressive reduction of bone turnover markers. Furthermore, increased marker levels after treatment discontinuation demonstrates preservation of bone remodelling capacity.
Conclusions
This study showed that zoledronic acid 4 mg once-yearly was well tolerated and effective in reducing biomarkers over 5 years. Detailed analysis of bone marker changes, however, suggests that this drug regimen causes insufficient reduction of remodelling activity in one third of patients.



Similar content being viewed by others
References
Mellström DD, Sörensen OH, Goemaere S et al (2004) Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 75:462–468
Bone HG, Hosking D, Devogelaer JP et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 350:1189–1199
Reid IR, Brown JP, Burckhardt P et al (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661
Garnero P, Sornay-Rendu E, Claustrat B et al (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J Bone Miner Res 15:1526–1536
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. A randomized controlled trial. JAMA 282(14):1344–1352, Oct 13
Bauer DC, Black DM, Garnero P et al (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. Fracture Intervention Trial Study Group. J Bone Miner Res 19:1250–1258
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Recker R, Stakkestad JA, Chesnut CH et al (2004) Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 34:890–899
Reginster JY, Christiansen C, Roux C et al (2001) Intermittent cyclic tiludronate in the treatment of osteoporosis. Osteoporosis Int 12:169–177
Acknowledgments
This study was supported by a research grant from Novartis Pharma AG, Basel, Switzerland. We are grateful to the other trial investigators and co-investigators: Dr Michael Hooper (Australia), Prof. Georg Leb (Austria), Dr. Maria Sääf (Sweden) and Dr. Hans Mallmin (Sweden), Christine Banville MD (Canada) and also study co-ordinators Evelyne Lejeune RN (Canada) and Suzanne Cardin RN (Canada). We wish to acknowledge Janet Douglas and Sarah Jackson from BioScience Communications for their editorial contributions.
Disclaimer
Supported by a research grant from Novartis Pharma AG, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devogelaer, J.P., Brown, J.P., Burckhardt, P. et al. Zoledronic acid efficacy and safety over five years in postmenopausal osteoporosis. Osteoporos Int 18, 1211–1218 (2007). https://doi.org/10.1007/s00198-007-0367-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0367-3