Abstract
Introduction and hypothesis
The objective was to evaluate the efficacy and safety of bulking agents compared with surgical methods for female stress urinary incontinence.
Methods
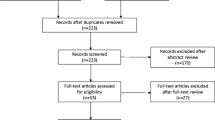
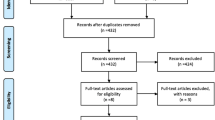
Inclusion and exclusion criteria: women with stress urinary incontinence. Bulking agents versus any surgical treatment as a comparison. Patients with other types of incontinence and treatment were excluded. Electronic databases (PubMed, MEDLINE, and the Cochrane Library) were searched from 2000 until 2021 to identify articles evaluating the effectiveness and safety of urethral bulking agents versus surgical methods. Risk-of-bias assessment tools recommended by the Cochrane Society were used to evaluate the risk of bias in the studies included.
Results
Six studies were included in the quantitative synthesis for a total of 710 patients. Our systematic review and meta-analysis showed that bulking agents are less effective than surgical procedures according to subjective improvement after treatment (RR = 0.70, 95% CI: 0.53 to 0.92, p = 0.01). There was no statistically significant difference between these two methods with regard to complications after the intervention (RR = 1.30, 95% CI: 0.30 to 5.66, p = 0.73).
Conclusion
The main limitation of this systematic review and meta-analysis was the absence of a common objective outcome measure to evaluate effectiveness. However, it shows that bulking agents are less effective than surgical procedures in subjective improvement. Safety analysis showed no significant difference between these methods. Hence, we believe that the first and final surgery is considered to be the best.







Similar content being viewed by others
References
Dyer O. Johnson and Johnson faces lawsuit over vaginal mesh devices. BMJ. 2016;353:i3045. https://doi.org/10.1136/bmj.i3045.
Keltie K, Elneil S, Monga A, et al. Complications following vaginal mesh procedures for stress urinary incontinence: an 8-year study of 92,246 women. Sci Rep. 2017;7:12015. https://doi.org/10.1038/s41598-017-11821-w.
Baessler K, Christmann-Schmid C, Maher C, Haya N, Crawford TJ, Brown J. Surgery for women with pelvic organ prolapse with or without stress urinary incontinence. Cochrane Database Syst Rev. 2018;8(8):CD013108. https://doi.org/10.1002/14651858.CD013108.
Morgan DM. Stress urinary incontinence in women: persistent/recurrent symptoms after surgical treatment. In: Brubaker L (Ed) UpToDate. Michigan: UpToDate; 2020.
Chapple C, Dmochowski R. Particulate versus non-particulate bulking agents in the treatment of stress urinary incontinence. Res Rep Urol. 2019;11:299–310. https://doi.org/10.2147/RRU.S220216
Medina CA, Costantini E, Petri E, Mourad S, Singla A, Rodríguez-Colorado S, et al. Evaluation and surgery for stress urinary incontinence: a FIGO working group report. Neurourol Urodyn. 2016;36(2):518–28. https://doi.org/10.1002/nau.22960.
Morling JR, McAllister DA, Agur W, Fischbacher CM, Glazener CM, Guerrero K, et al. Adverse events after first, single, mesh and non-mesh surgical procedures for stress urinary incontinence and pelvic organ prolapse in Scotland, 1997–2016: a population-based cohort study. Lancet. 2017;389(10069):629–40. https://doi.org/10.1016/S0140-6736(16)32572-7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Pivazyan L, Kasyan G, Grigoryan B, Pushkar D. Effectiveness of bulking agents vs. surgical methods in women with stress urinary incontinence: systematic review. PROSPERO 2021 CRD42021227128 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021227128
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (Eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
Mikkola T. TVT vs. polyacrylamide hydrogel injection for primary SUI—a randomized trial: AUA 2019: the American Urological Association’s 2019 Annual Meeting (AUA 2019), 3–6 May 2019, Chicago, Illinois, 2019.
Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, Tulokas S, Mikkola TS. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203(2):372–8. https://doi.org/10.1097/ju.0000000000000517.
Itkonen Freitas AM, Mikkola TS, Rahkola-Soisalo P, Tulokas S, Mentula M. Quality of life and sexual function after TVT surgery versus Bulkamid injection for primary stress urinary incontinence: 1 year results from a randomized clinical trial. Int Urogynecol J. 2021;32(3):595–601. https://doi.org/10.1007/s00192-020-04618-5.
Casteleijn FM, Enklaar RA, El Bouyahyaoui I, Jeffery S, Zwolsman SE, Roovers JWR. How cure rates drive patients’ preference for urethral bulking agent or mid-urethral sling surgery as therapy for stress urinary incontinence. Neurourol Urodyn. 2019;38(5):1384–91. https://doi.org/10.1002/nau.23997.
Mathieson R, Kippen R, Manning T, Brennan J. Stress urinary incontinence in the mesh complication era: current Australian trends. BJU Int. 2020;128(1):95-102. https://doi.org/10.1111/bju.15302.
De Vries AM, Wadhwa H, Huang J, Farag F, Heesakkers J, Kocjancic E. Complications of urethral bulking agents for stress urinary incontinence: an extensive review including case reports. Female Pelvic Med Reconstr Surg. 2017;24:392–8.
Kowalik CR, Casteleijn FM, van Eijndhoven HWF, Zwolsman SE, Roovers JWR. Results of an innovative bulking agent in patients with stress urinary incontinence who are not optimal candidates for mid-urethral sling surgery. Neurourol Urodyn. 2018;37(1):339–45.
Shortliffe LM, Freiha FS, Kessler R, et al. Treatment of urinary incontinence by periurethral implantation of glutaraldehyde cross-linked collagen. J Urol. 1989;141:538–41.
Corcos J, Fournier C. Periurethral collagen injection for the treatment of female stress urinary incontinence: 4-year follow-up results. Urology. 1999;54:815–8.
Appell RA, McGuire EJ, DeRidder PA, et al. Results for the multicenter study with injectable Gax collagen (abstract). J Urol. 1991;145:225A.
Herschorn SN. Early experience with intraurethral collagen injections for urinary incontinence. J Urol. 1992;148:1797–800.
Smith DN, Appell RA, Winters JC, et al. Collagen injection therapy for female intrinsic sphincteric deficiency. J Urol. 1997;157:1275–8.
Lee HN, Lee YS, Han JY, Jeong JY, Choo MS, Lee KS. Transurethral injection of bulking agent for treatment of failed mid-urethral sling procedures. Int Urogynecol J. 2010;21:1479–83.
Harriss JW, Iacovou JW, Lemberger RJ. Peri-urethral silicone microimplants (Macroplastique) for the treatment of genuine stress urinary incontinence. Br J Urol. 1996;78:722–8.
Kerr L. Bulking agents in the treatment of stress urinary incontinence: history, outcomes, patient populations, and reimbursement profile. Rev Urol. 2005;7:S3–S11.
Zullo MA, Plotti F, Bellati F, Muzii L, Angioli R, Panici PB. Transurethral polydimethylsiloxane implantation: a valid option for the treatment of stress urinary incontinence due to intrinsic sphincter deficiency without urethral hypermobility. J Urol. 2005;173:898–902.
Barranger E, Fritel X, Kadoch O, Liou Y, Pigne A. Results of transurethral injection of silicone microimplants for women with intrinsic sphincter deficiency. J Urol. 2000;164:1619–22.
Gumus I, Kaygusuz I, Derbent A, Simavli S, Kafali H. Effect of the Macroplastique implantation system for stress urinary incontinence in women with or without a history of an anti-incontinence operation. Int Urogynecol J. 2001;22:743–9.
Daly CME, Mathew J, Aloyscious J, et al. Urethral bulking agents: a retrospective review of primary versus salvage procedure outcomes. World J Urol. 2021;39(6):2107–12. https://doi.org/10.1007/s00345-020-03413-7.
Palmerola R, Peyronnet B, Rebolos M, et al. Trends in stress urinary incontinence surgery at a tertiary center: midurethral sling use following the AUGS/SUFU position statement. Urology 2019;131:71–6.
Stothers L, Goldenberg SL, Leone EF. Complications of periurethral collagen injection for stress urinary incontinence. J Urol. 1998;159:806–87.
Lee P, Kung R, Drutz H. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol. 2001;165(1):153–8. https://doi.org/10.1097/00005392-200101000-00037.
Lightner D, Calvosa C, Andersen R, Klimberg I, Brito C, Snyder J, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double-blind study of Durasphere. Urology. 2001;58(1):12–5. https://doi.org/10.1016/s0090-4295(01)01148-7.
Kunkle C, Hallock J, Hu X, Blomquist J, Thung S, Werner E. Cost utility analysis of urethral bulking agents versus midurethral sling in stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2015;21(3):154–9. https://doi.org/10.1097/spv.0000000000000173.
Corcos J, Collet JP, Shapiro S, Herschorn S, Radomski SB, Schick E, et al. Multicenter randomized clinical trial comparing surgery and collagen injections for treatment of female stress urinary incontinence. Urology. 2005;65(5):898–904. https://doi.org/10.1016/j.urology.2004.11.054.
Gaddi A, Guaderrama N, Bassiouni N, Bebchuk J, Whitcomb EL. Repeat midurethral sling compared with urethral bulking for recurrent stress urinary incontinence. Obstet Gynecol. 2014;123(6):1207–1212. https://doi.org/10.1097/AOG.0000000000000282. Erratum in: Obstet Gynecol. 2014 Oct;124(4):842.
Maher CF, O’Reilly BA, Dwyer PL, Carey MP, Cornish A, Schluter P. Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomised controlled trial. BJOG. 2005;112(6):797–801. https://doi.org/10.1111/j.1471-0528.2005.00547.x.
Wasenda EJ, Kirby AC, Lukacz ES, Nager CW. The female continence mechanism measured by high resolution manometry: urethral bulking versus midurethral sling. Neurourol Urodyn. 2018;37(5):1809–14. https://doi.org/10.1002/nau.23529.
Bach F, Toozs-Hobson P. An analysis of 1386 periurethral bulking procedures with comparison to 18,763 retropubic tapes. Post Reprod Health. 2020;26(2):71–8. https://doi.org/10.1177/2053369120924929.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. https://doi.org/10.1002/jrsm.1411.
ICS Standards 2020–2021. (2021). Retrieved 5 April 2021, from https://www.ics.org/members/shop/icsstandards20202021
U.S. Food and Drug Administration, FDA. U.S. Food and Drug Administration Executive Summary: reclassification of urogynecologic surgical mesh instrumentation. http://www.fda.gov/downloads/AdvisoryCommittees/ .
Kirchin V, Page T, Keegan PE, Atiemo KO, Cody JD, McClinton S, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7(7):CD003881. https://doi.org/10.1002/14651858.CD003881.pub4.
Araklitis G, Baines G, Da Silva AS, Rantell A, Robinson D, Cardozo L. Healthcare professional's choice for surgical management of stress urinary incontinence in a U.K. tertiary hospital. Eur J Obstet Gynecol Reprod Biol. 2021;263:7–14.https://doi.org/10.1016/j.ejogrb.2021.05.039
Author information
Authors and Affiliations
Contributions
Laura Pivazyan: data collection, Manuscript writing; George Kasyan: project development, manuscript editing; Bagrat Grigoryan: data collection, manuscript writing; Dmitry Pushkar: project development, manuscript editing.
Corresponding author
Ethics declarations
Financial disclaimer/conflicts of interest
None.
Other information
This systematic review has been registered in the PROSPERO international prospective register of systematic reviews by the National Institute for Health Research (NIHR). The registration number is PROSPERO 2021 CRD42021227128 [9].
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Registration
Registration number PROSPERO 2021 CRD42021227128
Rights and permissions
About this article
Cite this article
Pivazyan, L., Kasyan, G., Grigoryan, B. et al. Effectiveness and safety of bulking agents versus surgical methods in women with stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J 33, 777–787 (2022). https://doi.org/10.1007/s00192-021-04937-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04937-1