Abstract
Purpose
To assess whether invasive and non-invasive blood pressure (BP) monitoring allows the identification of patients who have responded to a fluid challenge, i.e., who have increased their cardiac output (CO).
Methods
Patients with signs of circulatory failure were prospectively included. Before and after a fluid challenge, CO and the mean of four intra-arterial and oscillometric brachial cuff BP measurements were collected. Fluid responsiveness was defined by an increase in CO ≥10 or ≥15 % in case of regular rhythm or arrhythmia, respectively.
Results
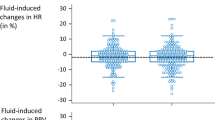
In 130 patients, the correlation between a fluid-induced increase in pulse pressure (Δ500mlPP) and fluid-induced increase in CO was weak and was similar for invasive and non-invasive measurements of BP: r² = 0.31 and r² = 0.29, respectively (both p < 0.001). For the identification of responders, invasive Δ500mlPP was associated with an area under the receiver-operating curve (AUC) of 0.82 (0.74–0.88), similar (p = 0.80) to that of non-invasive Δ500mlPP [AUC of 0.81 (0.73–0.87)]. Outside large gray zones of inconclusive values (5–23 % for invasive Δ500mlPP and 4–35 % for non-invasive Δ500mlPP, involving 35 and 48 % of patients, respectively), the detection of responsiveness or unresponsiveness to fluid was reliable. Cardiac arrhythmia did not impair the performance of invasive or non-invasive Δ500mlPP. Other BP-derived indices did not outperform Δ500mlPP.
Conclusions
As evidenced by large gray zones, BP-derived indices poorly reflected fluid responsiveness. However, in our deeply sedated population, a high increase in invasive pulse pressure (>23 %) or even in non-invasive pulse pressure (>35 %) reliably detected a response to fluid. In the absence of a marked increase in pulse pressure (<4–5 %), a response to fluid was unlikely.
Similar content being viewed by others
Introduction
In the critically ill, volume expansion is one of the most common interventions. It aims to increase the cardiac output (CO), and this occurs in “preload-dependent” patients.
A reliable detection of preload dependency (i.e., an accurate prediction of volume responsiveness) is often challenging, even with the use of recently developed tools such as the respiratory variations or the passive leg raising-derived indices. Indeed, predictive indices may be inapplicable [1–5], and a fluid challenge is then performed based on an informal “let’s give fluids and see what happens” approach [6, 7]. Ideally, this strategy requires measuring CO (or a robust surrogate) to ensure that CO has actually increased before considering another fluid challenge. CO measurement commonly relies on invasive indwelling devices or transthoracic ultrasonography, which may be limited by the patient's poor echogenicity, echograph availability and physicians’ skills in echocardiography.
As hypotension is a common trigger for volume expansion, clinicians are often tempted to use blood pressure (BP) changes as a very simple surrogate for CO changes: an increase in BP during fluid challenge is deemed to reflect an increase in CO, whereas, if the BP remains low, the patient has probably not responded. Indeed, if the arterial tree properties (arterial impedance) remain unchanged during the fluid challenge, one could expect that changes in BP and CO may be closely related [8]. Of note, studies addressing such a crucial issue of routine care are surprisingly scarce, and, in ICU patients, conflicting data exist [9–12]. None of the studies examined non-invasive measurements of BP as a surrogate of CO changes. Indeed, in the ICU, BP is commonly measured through an intra-arterial catheter but also with an automated brachial cuff, for instance before the insertion of the intra-arterial catheter [13, 14].
We aimed at assessing, in a large population of patients with acute circulatory failure, whether changes in BP (measured either invasively or non-invasively) are correlated with changes in CO during a fluid challenge and allow the identification of patients who actually responded to fluid. As diagnostic tests are rarely binary in real-life practice, the extent of inconclusive values (“gray zone”) of the tested indices was also calculated.
Patients and methods
Patients
Patients of three medical ICUs [Hôpital Bichat-Claude Bernard (Paris), Centre Hospitalier Régional Universitaire of Tours, Centre Hospitalier Régional of Orléans, France] were included over an 18-month period, either after obtaining written informed consent from a relative, followed by delayed consent from the patient him/herself when possible, or after emergency enrollment followed by delayed consent, as approved by the regional ethics board. These patients were part of a prospectively acquired database that had already yielded published works [2, 5, 15, 16].
We included adults suffering from acute circulatory failure, defined by the presence of at least one criterion among systolic BP <90 mmHg, mean BP <65 mmHg, vasopressor infusion, skin mottling, lactatemia >2.5 mmol/l and urine output <0.5 ml/kg/h (with no other evident reason for oliguria than systemic hypoperfusion). The attending physician agreed that a fluid challenge had to be considered.
Patients were not included in case of diuretic treatment within the last 4 h, uncontrolled hemorrhage or brain death. Patients were also not included or excluded in case of risk of fluid loading-induced life-threatening hypoxemia: PaO2/FiO2 <70 mmHg, body weight indexed extravascular lung water (EVLWi) >22 ml.kg−1 (PiCCO™ system: Pulsion Medical Systems AG™, Munich, Germany), transmural pulmonary artery occlusion pressure (PAOPtm) >22 mmHg (pulmonary artery catheter: Edwards Lifesciences™, Irvine, CA) [17] or a 5 % decrease in pulse oxymetry during volume expansion. Mechanical ventilation, vasoactive therapy, sedation and paralysis were not modified.
Measurements
Heart rate, BP and CO were measured at baseline and immediately after a 500-ml gelatin infusion over 30 min. After 300 ml, hemodynamic measurements were also performed to ensure that volume expansion was well tolerated. CO was measured through end-expiratory injection of 10 or 15 ml (transcardiac and transpulmonary thermodilution, respectively) of an iced dextrose solution [using a closed injection system with in-line temperature measurement: CO-set+™ system (Edwards Lifesciences™, Irvine, CA) or included in the PiCCO™ system]. Three consecutive end-expiratory measurements within 10 % (if not, seven measurements) were averaged.
The correct placement of the pulmonary artery catheter was ascertained by visualization of concordant waveforms and calculation of the respiratory changes in PAOP-to-respiratory changes in the PAP ratio [18].
BP was measured using (1) an intra-arterial catheter (pressure transducer zeroed at the level of the mid-axillary line, T100209A, Edwards Lifesciences, Irvine, CA, or PiCCO, Pulsion Medical Systems AG, Munich, Germany) and (2) an oscillometric automated brachial cuff (SC9000 monitor, Siemens AG, Munich, Germany, or Intellivue MP70 monitor, Philips Medical Systems, Best, The Netherlands, depending on the study center), with an adequate cuff size [19], placed on the arm opposite to an eventual radial artery catheter.
Four pairs of invasive and non-invasive BP measurements were performed (at 30- to 60-s intervals).
In patients with regular cardiac rhythm and fully adapted to the ventilator, respiratory pulse pressure variations (PPV) were extracted from the invasive BP waveform: off-line, on high-resolution paper tracings, including airway waveform, and after their numerical enlargement, PPV was calculated (by an observer blinded to other hemodynamic data) [20] and averaged over three consecutive respiratory cycles.
An echocardiography (within 6 h of measurements) looked for valvular regurgitations and intra-cardiac shunts, which could have distorted the thermodilution measurements.
Statistical analysis
Studied parameters
The mean of the four consecutive measurements of BP was considered. Fluid-induced variation (in %) of systolic (Δ500mlSBP), mean (Δ500mlMBP), diastolic (Δ500mlDBP) and pulse BP (Δ500mlPP) were calculated. Their respective relationship with fluid-induced variation of CO (Δ500mlCO) was examined.
In patients with regular rhythm and no respiratory effort, the fluid-induced decrease in PPV was calculated (Δ500mlPPV).
Definition of fluid responsiveness
As proposed by Ostergaard et al., patients were classified as responders if volume expansion increased CO by more than 10 or 15 % in case of regular rhythm or arrhythmia, respectively [21].
Statistical tests
Correlations were assessed by linear regression. For each parameter, we calculated the area under the receiver operating characteristic curve (AUC) for the detection of fluid responsiveness and determined positive and negative likelihood ratios (LR).
The whole population was split into subgroups according to the presence of septic shock, the site of the intra-arterial catheter (radial or femoral artery), patient age (above/below median), baseline mean BP (above/below 70 mmHg) and cardiac rhythm (regular/arrhythmic). The corresponding AUCs were compared [22]. Other variables [expressed as mean ± SD or n (%)] were compared using Student's t test and Fisher's exact test (between responders and nonresponders), and the paired Student's t-test (within each patient). All statistical tests were two-tailed, performed using MedCalc® (Mariakerke, Belgium) and Statview® (SAS Institute, Cary, NC). p < 0.05 was considered significant.
Gray zone definition
A minimal value of 5 for the positive LR (or a maximal value 0.2 for the negative LR) is required to consider that a test has “good” positive (or negative, respectively) diagnostic performance [23]. Therefore, we defined the gray zone (i.e., the inconclusive values) as the continuous scale of thresholds associated with a negative LR >0.2 and a positive LR <5.
Results
Among 130 included patients (Tables 1, 2), 48 (37 %) responded to volume expansion.
In three patients, volume expansion was interrupted after 300 ml because of intolerance (6 % drop in SpO2, sharp increase in PAOP or in EVLWi >22 ml/kg), and data after 300 ml were used for analysis.
Relationship between fluid-induced changes in BP and changes in CO
The correlation between Δ500mlPP and Δ500mlCO was similar but weak for invasive and non-invasive measurements of BP: r² = 0.31 and r² = 0.29, respectively (both p < 0.001, Fig. 1).
A significant correlation was also found for invasive Δ500mlSBP and Δ500mlMBP but not for invasive Δ500mlDBP. Similar results were found for non-invasive indices (ESM 1).
No index outperformed Δ500mlPP. For the 91 patients with regular rhythm and no inspiratory effort, the relationship between Δ500mlPPV and Δ500mlCO (r² = 0.07, p = 0.009) was weaker (p = 0.003) than between invasive Δ500mlPP and Δ500mlCO (r² = 0.35, p < 0.001).
Identification of patients having responded to volume expansion
invasive Δ500mlPP was associated with an AUC of 0.82 (0.74–0.88), similar (p = 0.8) to that of non-invasive Δ500mlPP [AUC of 0.81 (0.73–0.87)] (Fig. 2).
Identification of responders to fluid challenge with fluid-induced changes in pulse pressure (Δ500mlPP) and in systolic blood pressure (Δ500mlSBP). Fluid responsiveness was defined by a fluid-induced increase in cardiac output ≥10 % in case of regular rhythm and ≥15 % in case of arrhythmia [21]
The gray zone of invasive Δ500mlPP ranged from 5 to 23 %: an invasive Δ500mlPP of 5 % was associated with a negative LR of 0.17 (0.06–0.4) [sensitivity of 92 % (80–98 %)], whereas an invasive Δ500mlPP of 23 % was associated with a positive LR of 5.1 (2.8–9.5) [specificity of 88 % (79–94 %)]. In other words, in case of invasive Δ500mlPP below 5 %, there was good identification of nonresponse to fluid challenge, and if invasive Δ500mlPP was >23 %, there was good identification of response [23]. Values comprised in the gray zone were, per definition, inconclusive, and involved 45 (35 %) patients (including 14 responders) (ESM 4).
For non-invasive Δ500mlPP, the gray zone was 4–35 % [involving 62 (48 %) patients]: the negative LR was 0.19 (0.07–0.5) [sensitivity of 92 % (80–98 %)] for the 4 % threshold and the positive LR 5.1 (2.4–11.2) [specificity of 91 % (83–97 %)] for the 35 % threshold.
invasive and non-invasive Δ500mlSBPs were associated with the same AUC of 0.79 (0.71–0.86) (Fig. 2). The AUC for invasive and non-invasive Δ500mlMBP and Δ500mlDBP did not exceed 0.73, which was significantly lower than that of Δ500mlPP (p < 0.05 for all comparisons).
In patients with regular rhythm and no inspiratory effort, the AUC for Δ500mlPPV (0.66) was significantly lower than that of invasive Δ500mlPP (0.82) or invasive Δ500mlSBP (0.79) (all p < 0.05, ESM 2).
Influence of clinical factors
As compared with patients with regular rhythm (n = 106), the arrhythmic status (n = 24) did not impair the performance of Δ500mlPP, even for non-invasive measurements (ESM 3).
In addition, the AUC for Δ500mlPP (either invasive or non-invasive) was similar when splitting the whole population into two subgroups according to age, baseline BP, the presence of a septic shock (n = 58), the site of the intra-arterial catheter or the model of the oscillometric device (ESM 3).
Influence of methodological factors
Similar results were found when considering fluid-induced changes in stroke volume rather than in CO: r² = 0.38 and 0.31, AUC of 0.80 (0.72–0.86) and 0.82 (0.75–0.88), for invasive and non-invasive Δ500mlPP, respectively.
Finally, analyzing the first BP measurement at each study phase rather than the mean of 4 consecutive measurements did not impair the performance of invasive Δ500mlPP [same AUC of 0.82 (0.74–0.88)]. A similar result was found for non-invasive Δ500mlPP [AUC of 0.78 (0.70–0.85) vs. 0.81 (0.73–0.87), p = 0.2], even in case of arrhythmia [AUC of 0.79 (0.58–0.93) vs. 0.78 (0.69–0.85), p = 0.9].
Discussion
The first main finding of this study is that, as reflected by large gray zones, only a very low/high increase in pulse pressure reliably reflected CO unresponsiveness/responsiveness to fluid. Second, non-invasive measurements of BP did not show worse performance than invasive measurements for this purpose, even in case of arrhythmia.
Pathophysiological considerations
The correlation between invasive Δ500mlPP and Δ500mlCO was significant but weak (r² = 0.31). Beyond possible errors in the measurement of BP and even of CO, a fluid-induced change in the vasomotor tone probably explains why Δ500mlPP did not parallel Δ500mlCO more strongly. Indeed, a change in total arterial compliance (which equals stroke volume/aortic pulse pressure) [24] alters the relationship between stroke volume and pulse pressure. It may also change pulse wave transmission and reflection characteristics and impact BP [25]. These pathophysiological considerations are of utmost importance as, during acute circulatory failure, fluid-induced changes in vascular tone are expected or even hoped for. Hence, the fact that the clinician may often be misled when paying attention to changes in BP in order to estimate how CO has changed is rather obvious and is confirmed by the weak correlations observed.
Of note, the tested indices did not parallel stroke volume changes better than CO changes. As stroke volume and CO are linked by heart rate, this may be due to a lack of marked change in heart rate during fluid challenge (even if this change reached statistical significance, Table 2).
As pulse pressure is directly related to stroke volume (and then to CO) [25, 26], the fact that Δ500mlPP performed better than Δ500mlMAP was expected.
Comparison with other studies
First of all, it is surprising to notice that the available data about such a common practice are scarce. Indeed, including the perioperative literature, only a few specific studies have addressed the reliability of changes in BP during a fluid challenge as a surrogate for changes in CO, and none have assessed non-invasive measurements of BP for this purpose. In comparison with our findings, two of these studies reported a similar relationship between invasive Δ500mlPP and Δ500mlCO (r² of 0.25–0.35) [9, 10], whereas one study in the perioperative setting found a less close correlation (r² = 0.03) [12], and another one (including only 51 patients) reported no correlation at all [11]. These discrepancies may be explained by different arterial tree characteristics [27]. In our population, the fluid-induced changes in arterial tone were sufficiently mild to let Δ500mlPP parallel Δ500mlCO. This may be due to deep sedation (Ramsay scale = 5–6) [28], which could have partly impaired the sympathetic/parasympathetic mechanisms of BP autoregulation, thus limiting fluid-induced changes in vasomotor tone [25].
In our population, contrary to the perioperative study by Le Manach et al. [12], Δ500mlPPV did not outperform the other BP-derived indices. This finding may be related to measurement inaccuracies. Our low baseline mean PPV is noteworthy, probably related to the low tidal volume [29], low heart-to-respiratory rate ratio [30] and previous resuscitation. Hence, low baseline PPV comes along with low Δ500mlPPV, i.e., a low signal-to-noise ratio exposed to measurement inaccuracies in particular with our manual method of calculation of PPV on paper tracings, a less accurate method than an automated one. However, one should keep in mind that, contrary to the other BP-derived indices and contrary to the perioperative setting, Δ500mlPPV is far from an ubiquitous index as it only applies to a few ICU patients: those with regular cardiac rhythm and those who are so deeply sedated they do not trigger the ventilator [31].
Strengths of this study
To the best of our knowledge, our study is the first assessing non-invasive measurements of BP for tracking fluid-induced changes in CO. Of note, most of the intensivists often rely on these automated brachial cuff measurements for their decision making (e.g., before the insertion of an arterial line) [13]. Indeed, a fluid challenge is often prescribed because hypotension is displayed via non-invasive measurements, and tracking its benefit then also relies on these non-invasive measurements. Again, surprisingly, no studies addressed this important issue. For invasive measurements of BP, data are also scarce [9–12].
Furthermore, we provided helpful gray zone boundaries for Δ500mlPP rather than a single universal cutoff of poor clinical help (because of positive LR <5 and negative LR >0.2) [23].
Last, a non-negligible proportion of arrhythmic patients were analyzed, whereas, in similar studies, arrhythmia was an exclusion criterion [9, 12, 32] or not mentioned [10, 11]. Of note, Δ500mlPP did not perform worse during arrhythmia, even with non-invasive measurements (and even considering one non-invasive reading per phase rather than the mean of four consecutive readings). This finding is noteworthy as oscillometric brachial cuff measurements are classically deemed to be flawed in case of arrhythmia [33, 34].
Study limitations
First, along with errors in invasive [25, 35] and non-invasive measurements of BP [36, 37], our thermodilution measurements of CO are also exposed to errors [21]. With more accurate devices, the relationship between BP and CO may have been stronger. Nevertheless, we measured BP with the commonly used devices, and our CO measurements were the average of several rigorous readings. As we focused on changes in CO rather than absolute values, the use of two different thermodilution methods for CO determination does not appear to be a limitation.
Second, the infusion of 500 ml gelatin over 30 min could have been an insufficient fluid stimulus. On top of fluid-induced changes in CO, this lengthy duration may cause exposure to colloid transfer outside the intravascular compartment resulting in changes in vasomotor tone. A faster fluid challenge could have yielded a different relationship between BP and CO.
Third, our inclusion criteria allowed the inclusion of patients with signs of shock but not suffering from hypotension and not receiving vasopressive agents. In this situation, the clinician is likely to look for an increase in urine output, an improvement in skin hypoperfusion and a decrease in heart rate or arterial lactate (depending on the sign that triggered the fluid challenge) rather than paying attention to an increase in BP to assess whether the patient responded to fluid. However, only four patients received a fluid challenge not triggered by hypotension or vasopressive agent infusion. Their exclusion from the analysis did not change our findings.
Fourth, we did not collect any data about the resolution of clinical or biological signs of poor tissue perfusion after the fluid challenge. Therefore, we can only make the assumption that if Δ500mlPP fell into the inconclusive gray zone, the clinician should rely on other means to assess the clinical benefit of the fluid challenge: e.g., increase in urine output, vanishing of skin mottling and correction of a lactic acidosis.
Finally, we did not collect the amount of fluid infused before inclusion. However, previous large fluid resuscitation was very likely as reflected by the high mean baseline CVP (11 mmHg), low mean baseline PPV (6 %) and low rate of fluid responders (37 %). However, it is precisely in these already resuscitated patients still exhibiting signs of poor tissue perfusion that assessing the effects of a fluid challenge is important before considering further volume expansion or another cardiovascular therapy. Caution should be exercised when extrapolating our findings to the very first hours of shock resuscitation.
Clinical implications
During fluid challenge, only an important increase in pulse pressure (Δ500mlPP > 23 % for invasive and >35 % for non-invasive) reliably reflects that the patient has responded. Conversely, a low Δ500mlPP (<4–5 %) is necessary to reliably rule out a response to fluid, making an additional fluid challenge useless or even harmful.
The second main finding is that invasive BP-derived indices did not outperform non-invasive indices, even in case of arrhythmia. Importantly, half of our patients fell in the gray zone of non-invasive Δ500mlPP, whereas, for invasive Δ500mlPP, it only involved one third.
Conclusions
It is impossible to identify fluid challenge-induced changes in cardiac output in one-third to one half of patients solely via blood pressure monitoring. Only very low/high increases in arterial pulse pressure, even measured non-invasively, reliably reflected unresponsiveness/responsiveness to fluid.
References
Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B (2011) Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 115:231–241
Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, Mercier E, Wolff M, Regnier B, Boulain T (2011) Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care 15:R85
Mahjoub Y, Pila C, Friggeri A, Zogheib E, Lobjoie E, Tinturier F, Galy C, Slama M, Dupont H (2009) Assessing fluid responsiveness in critically ill patients: false-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med 37:2570–2575
Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, Tinturier F, Slama M, Dupont H (2010) The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med 38:1824–1829
Lakhal K, Ehrmann S, Runge I, Benzekri-Lefevre D, Legras A, Dequin PF, Mercier E, Wolff M, Regnier B, Boulain T (2010) Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive Care Med 36:940–948
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med 34:1333–1337
Cecconi M, Parsons AK, Rhodes A (2011) What is a fluid challenge? Curr Opin Crit Care 17:290–295
Convertino VA, Cooke WH, Holcomb JB (2006) Arterial pulse pressure and its association with reduced stroke volume during progressive central hypovolemia. J Trauma 61:629–634
Dufour N, Chemla D, Teboul JL, Monnet X, Richard C, Osman D (2011) Changes in pulse pressure following fluid loading: a comparison between aortic root (non-invasive tonometry) and femoral artery (invasive recordings). Intensive Care Med 37:942–949
Monnet X, Letierce A, Hamzaoui O, Chemla D, Anguel N, Osman D, Richard C, Teboul JL (2011) Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine. Crit Care Med 39:1394–1399
Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL (2012) Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med 38:422–428
Le Manach Y, Hofer CK, Lehot JJ, Vallet B, Goarin JP, Tavernier B, Cannesson M (2012) Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology 117:1165–1174
Chatterjee A, DePriest K, Blair R, Bowton D, Chin R (2010) Results of a survey of blood pressure monitoring by intensivists in critically ill patients: a preliminary study. Crit Care Med 38:2335–2338
Lehman LW, Saeed M, Talmor D, Mark R, Malhotra A (2013) Methods of blood pressure measurement in the ICU. Crit Care Med 41:34–40
Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, Mercier E, Wolff M, Regnier B, Boulain T (2012) Brachial cuff measurements of blood pressure during passive leg raising for fluid responsiveness prediction. Ann Fr Anesth Reanim 31:e67–e72
Lakhal K, Ehrmann S, Runge I, Legras A, Dequin PF, Mercier E, Wolff M, Regnier B, Boulain T (2009) Tracking hypotension and dynamic changes in arterial blood pressure with brachial cuff measurements. Anesth Analg 109:494–501
Teboul JL, Pinsky MR, Mercat A, Anguel N, Bernardin G, Achard JM, Boulain T, Richard C (2000) Estimating cardiac filling pressure in mechanically ventilated patients with hyperinflation. Crit Care Med 28:3631–3636
Teboul JL, Besbes M, Andrivet P, Axler O, Douguet D, Zelter M, Lemaire F, Brun-Buisson C (1992) A bedside index assessing the reliability of pulmonary occlusion pressure measurements during mechanical ventilation with positive end-expiratory pressure. J Crit Care 7:22–29
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ (2005) Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111:697–716
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Ostergaard M, Nilsson LB, Nilsson JC, Rasmussen JP, Berthelsen PG (2005) Precision of bolus thermodilution cardiac output measurements in patients with atrial fibrillation. Acta Anaesthesiol Scand 49:366–372
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Grimes DA, Schulz KF (2005) Refining clinical diagnosis with likelihood ratios. Lancet 365:1500–1505
Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y (1998) Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 274:H500–H505
Lamia B, Chemla D, Richard C, Teboul JL (2005) Clinical review: interpretation of arterial pressure wave in shock states. Crit Care 9:601–606
Cecconi M, Rhodes A (2011) Pulse pressure: more than 100 years of changes in stroke volume. Intensive Care Med 37:898–900
Antonelli M, Bonten M, Chastre J, Citerio G, Conti G, Curtis JR, De Backer D, Hedenstierna G, Joannidis M, Macrae D, Mancebo J, Maggiore SM, Mebazaa A, Preiser JC, Rocco P, Timsit JF, Wernerman J, Zhang H (2012) Year in review in Intensive Care Medicine 2011. II. Cardiovascular, infections, pneumonia and sepsis, critical care organization and outcome, education, ultrasonography, metabolism and coagulation. Intensive Care Med 38:345–358
Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br Med J 2:656–659
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31:517–523
De Backer D, Taccone FS, Holsten R, Ibrahimi F, Vincent JL (2009) Influence of respiratory rate on stroke volume variation in mechanically ventilated patients. Anesthesiology 110:1092–1097
Pinsky MR (2007) Heart-lung interactions. Curr Opin Crit Care 13:528–531
Monge Garcia MI, Gil Cano A, Gracia Romero M (2011) Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care 15:R15
Farsky S, Benova K, Krausova D, Sirotiakova J, Vysocanova P (2011) Clinical blood pressure measurement verification when comparing a Tensoval duo control device with a mercury sphygmomanometer in patients suffering from atrial fibrillation. Blood Press Monit 16:252–257
Anastas ZM, Jimerson E, Garolis S (2008) Comparison of noninvasive blood pressure measurements in patients with atrial fibrillation. J Cardiovasc Nurs 23:519–524 quiz 525–516
Ercole A (2006) Attenuation in invasive blood pressure measurement systems. Br J Anaesth 96:560–562
O’Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, Imai Y, Wang J, Mengden T, Shennan A (2010) European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 15:23–38
van Montfrans GA (2001) Oscillometric blood pressure measurement: progress and problems. Blood Press Monit 6:287–290
Acknowledgments
The authors sincerely acknowledge the help of all the staff of the participating intensive care units as well as Ms. Andrea Pritzker for the English editorial assistance.
Regional grant: Projet Hospitalier de Recherche Clinique (PHRC no. R10-5), centre hospitalier d’Orléans, France, September 2004.
Conflicts of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakhal, K., Ehrmann, S., Perrotin, D. et al. Fluid challenge: tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive). Intensive Care Med 39, 1953–1962 (2013). https://doi.org/10.1007/s00134-013-3086-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3086-6