Abstract
Background
Current medical knowledge lacks specific information regarding creatine kinase (CK) elevation in influenza A pH1N1 (2009) infection.
Objectives
Primary endpoints were correlation between CK at intensive care unit (ICU) admission and ICU mortality. Secondary endpoints were ICU length of stay (LOS), mechanical ventilation (MV), and requirement of renal replacement techniques (RRT).
Materials and methods
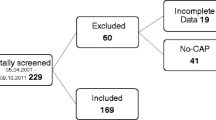
A prospective multicenter register included all adults admitted for severe acute respiratory insufficiency (SARI) with confirmed pH1N1 in 148 ICUs. Clinical data including demographics, comorbidities, laboratory information, organ involvement, and prognostic data were registered. Post hoc classification of subjects was determined according to CK level. Data are expressed as median (interquartile range).
Results
Five hundred and five (505) patients were evaluable. Global ICU mortality was 17.8 % without documented differences between breakpoints. CK ≥500 UI/L was documented in 23.8 % of ICU admissions, being associated with greater renal dysfunction: acute kidney injury (AKI) was more frequent (26.1 versus 17.1 %, p < 0.05) and twofold requirement of RRT [11 versus 5.6 %, p < 0.05; odds ratio (OR) = 2.09 (95 % confidence interval [CI] 1.01–4.32)]. Increase of CK ≥1,000 UI/L was associated with two or more quadrant involvement on chest X-ray (63.2 versus 40.2 %, p < 0.01) and increased intubation risk (73.9 versus 56.7 %, p = 0.07) and duration of mechanical ventilation (median 15 days versus 11 days, p < 0.01). As a result, CK ≥1,000 UI/L was associated with 5 extra days of ICU and hospital LOS.
Conclusions
CK is a biomarker of severity in pH1N1 infection. Elevation of CK was associated with more complications and increased ICU LOS and healthcare resources.
Similar content being viewed by others
Introduction
pH1N1 (2009 pandemic influenza A) presented with substantial pulmonary involvement; however, extrapulmonary complications were not uncommon and represented an additional contribution to mortality [1–3]. Risk factors for poor outcome included multiple-organ dysfunction syndrome and requirement of mechanical ventilation or renal replacement therapies [4–6]; indeed, stage III acute kidney injury (AKI) has proved to be an independent risk factor for mortality [7].
Association between rhabdomyolysis and influenza A and B virus infection has been previously described [8, 9]. Proposed pathogenic mechanism includes viral invasion, following viral toxin and host immune-mediated cytokine production, which might be involved in muscular injury [9, 10]. However, to date, true pathogenesis remains unknown.
The purpose of this study is to assess if increased creatine kinase (CK) was related to worse global, renal, and respiratory outcomes in critically ill patients with pH1N1 infection, and if it could serve as a biomarker of severity. The primary endpoint was intensive care unit (ICU) mortality. Secondary endpoints were correlation between CK levels and ICU length of stay (LOS), mechanical ventilation (MV), and requirement of renal replacement techniques (RRT). Our hypothesis was that mild elevations of CK (below those usually seen with rhabdomyolysis) in pH1N1 infection were associated with AKI and severe respiratory failure, increasing ICU LOS and ICU mortality.
Materials and methods
The study was conducted in 148 Spanish intensive care units, from June 2009 to February 2010. CK breakpoints were 300, 500, 1,000, 2,500, 3,000, 4,000, and 10,000 UI/L. Given its observational structure, informed consent was waived. Data were obtained using a voluntary standardized registry by investigators of the Infectious Disease Working Group of the Spanish Society of Intensive Care Medicine (GTEI-SEMICYUC). The Ethics Committee of all hospitals approved this study.
We included all patients admitted to the ICU for severe acute respiratory insufficiency (SARI) with clinical suspicion of pH1N1 corresponding to the World Health Organization (WHO) case definitions [11] and microbiological confirmation. Clinical suspicion was defined by febrile (>38 °C) acute illness, respiratory symptoms (consistent with cough, sore throat), myalgia or influenza-like illness. Patients under 15 years were excluded. Microbiological confirmation was documented by real-time polymerase chain reaction (RT-PCR) (according to Centers for Disease Control and Prevention guidelines) [12] or viral culture, which were performed at the corresponding healthcare facility (pH1N1 testing was performed in each institution, or centralized in a reference laboratory when local resources were not available). Specimens were obtained from nasopharyngeal swabs or respiratory secretions in intubated patients. All patient management was determined by an attending physician, from the decision of ICU admission to ICU discharge.
Clinical data consisting of baseline demographics, comorbidities, pulmonary involvement, laboratory findings, and severity were registered at time of ICU admission, whereas organ involvement, treatment received, and prognostic data were registered during ICU and hospital stay. Demographic data included age and sex. Comorbidities data included presence or absence of diabetes mellitus, chronic kidney disease, cardiac heart failure, chronic obstructive pulmonary disease, asthma, pregnancy, body mass index (BMI) >30 kg/m2, BMI >40 kg/m2, HIV/AIDS, autoimmune disorder, hematological disorder, and neuromuscular disorder. Pulmonary data included primary diagnosis at time of ICU admission, pneumonia (viral or bacterial), chronic obstructive pulmonary disease (COPD) or asthma exacerbation, and bacterial co-infection. Laboratory data included creatine kinase, urea, and creatinine. Severity of the disease was assessed at ICU admission by Sequential Organ Failure Assessment (SOFA) [13].
Normal CK levels found in medical references range in men from 52–55 to 170–294 UI/L and in women from 39–45 to 135–238 UI/L [14, 15]. Therefore, we defined elevated CK values as those greater than or equal to 300 UI/L. Organ involvement was classified into two categories: renal and respiratory. Respiratory compromise was defined by the number of affected quadrants on chest radiograph, the number of patients who required mechanical ventilation, and duration of MV. Definition of pneumonia was established based on the Infectious Disease Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults [16]. Primary viral pneumonia was defined as illness presenting with acute respiratory distress and unequivocal alveolar opacities involving two or more lobes with negative respiratory and blood bacterial cultures during the acute phase of influenza virus. Infections occurring later were considered nosocomial. Renal dysfunction (AKI) was defined as increase of serum creatinine of 50 % or 0.3 mg/dL from baseline values within 48 h [in accordance with Acute Kidney Injury Network (AKIN) definitions] [17], or by the requirement of renal replacement therapies, which included either dialysis or continuous veno-venous hemofiltration (CVVHF).
Statistical analysis
Due to the non-Gaussian distribution of the data, discrete variables are reported as frequency (%) and continuous variables as median (interquartile range, IQR). Odds ratio (OR) is presented with 95 % confidence interval (CI). Statistical analysis was performed using SPSS for Mac, version 18 (SPSS, Chicago, IL, USA). Univariate analysis was performed using Pearson’s χ 2 to calculate the two-tailed p value. Mann–Whitney U test was performed on quantitative variables. Statistical significance was defined by p < 0.05.
Results
Five hundred and five patients were evaluated. Patients were predominantly young (43 years), with no gender predominance. The majority had at least one underlying disease: 20 % were overweight, and between 10 and 16 % had type 2 diabetes mellitus, COPD, or asthma. Less than 5 % had chronic kidney disease. Median SOFA was 4 points. Normal CK values were documented in 64.7 %. CK was higher than 300, 500, 1,000, 2,500, 3,000 and 4,000 in 35.2, 23.8, 17.8, 4.8, 4.6, and 2.8 %, respectively. Only 1.2 % had CK levels above 10,000 UI/L at ICU admission (Table 1; Fig. 1). Median baseline values of renal function ranged within or were slightly above normal values. The majority (84 %) presented with a severe acute respiratory infection secondary to pneumonia at ICU admission. See Table 2 for clinical and laboratory characteristics of the patients. Global ICU mortality was 17.2 % (90 patients), with no difference when analyzed at the 300 and 500 UI/L breakpoints (p = 0.5 and 0.49, respectively; Table 3).
In patients with CK ≥500 UI/L, AKI was more frequent [26.1 versus 17.1 %, p < 0.05; OR = 1.7 (95 % CI 1.04–2.77)], with twofold requirement for RRT [9.3 versus 4.5 %, p < 0.05; OR = 2.17 (95 % CI 1.00–4.78)] (Table 4). For CK level ≥2,500 UI/L, a threefold higher requirement for renal replacement techniques was documented [17.4 versus 6.4 %; OR = 3.1 (95 % CI = 0.99–9.68)], although this did not reach statistical significance (p < 0.07).
Greater respiratory impairment was seen in patients with CK ≥1,000 UI/L. They were more likely to have two or more quadrants affected on chest radiograph (63.2 versus 40.25 %, p < 0.01), significantly longer duration of mechanical ventilation [median (IQR) 15 (8.5–27) days versus 11 (6–17.8) days (p < 0.01)], and more frequent intubation (73.9 versus 56.7 %), although this finding did not reach statistical significance (p = 0.07) (Table 5). Patients with CK ≥1,000 UI/L also had significantly (p = 0.01) longer median ICU length of stay [13 (6–25) days versus 8 (4–16) days] and median hospital length of stay [15 (8–25) days versus 20 (13–34) days] (Table 6).
Discussion
The results of our study suggest that CK level is a biomarker of severe pH1N1 infection. Interestingly, even though no difference in mortality was observed, we found that slight elevations were associated with increased pulmonary and kidney complications, likewise increased length of stay (both ICU and hospital). Furthermore, starting from CK >500 UI/L, a breakpoint considerably close to normal upper values, significant associations with worse renal outcomes were documented. These included higher occurrence of AKI, as well as more frequent need for RRT, which progressively increased with rising CK values. Greater pulmonary injury was observed from CK >1,000 UI/L, with 50 % more involvement in chest radiograph in addition to more prolonged mechanical ventilation (Fig. 2).
Although some isolated case reports of pH1N1-associated rhabdomyolysis have been published [18–22], uncertainty still remains regarding the clinical implications of elevated creatine kinase levels. While the association of elevated CK with requirement for RRT has been documented at CK level around 11,000 UI/L in several pH1N1 case reports [23, 24], others have failed to demonstrate a need for RRT, even at levels of CK of 16,000 UI/L and above [22, 25, 26]. A series of case reports showed elevated levels (1,000–5,000 UI/L) of CK in severe presentations of pH1N1 infection [27], and one case report showed AKI due to pH1N1-induced rhabdomyolysis (with CK levels above 40,000 UI/L) [28]. To our knowledge, this is the first large series that evaluates prospectively the relationship between CK levels and clinical outcomes in a large cohort of patients with pH1N1 infection.
Moreover, there is not a validated CK breakpoint for diagnosis of rhabdomyolysis. Literature [29–32] defines rhabdomyolysis by elevation of CK values above 10,000 UI/L (almost exclusively of the skeletal muscle fraction, MM) which is usually accompanied by elevation of other enzymes (aldolase, lactate dehydrogenase (LDH), aspartate aminotransferase, and alanine aminotransferase). Likewise, medical literature lacks specific information and evidence for CK values regarding rhabdomyolysis and its clinical course. The term hyperCKemia [33] refers to a less severe form of rhabdomyolysis, secondary to chronic or intermittent muscle destruction characterized by mild elevation of creatine kinase, which is usually asymptomatic, and without renal impairment. Kidney failure has been associated with levels of CK above 20,000 UI/L in patients without concomitant significant disease (13–50 % of cases), however, lower cutoff points (5,000 UI/L) have been reported in patients with dehydration, sepsis or acidosis. In our study, slight CK elevations within the range of hyperCKemia at ICU admission (which usually are not taken into consideration in the management of patients) were associated with kidney and lung complications and worse outcomes. This raises the question of whether the standard concept of pathological values of CK associated with rhabdomyolysis may not apply in other contexts and whether the definition of new risk-based breakpoints would have greater relevance. Also of interest, we found an association between CK levels and severity of respiratory failure and duration of mechanical ventilation.
Limitations are inherent to the design of this study, particularly the lack of measurement of CK levels during ICU stay to assess the exact threshold that might cause AKI in pH1N1 infection and therefore establish which CK levels were related to increased risk for developing AKI and which levels are crucial for AKI. Decisions for intubation or renal replacement therapy were not standardized, and this weakness may limit generalization. Also, creatine kinase MB fraction was not determined, so cardiac origin of CK elevation could not be ruled out. Likewise, other biomarkers such as aldolase or LDH were not recorded.
In summary, our findings suggest that CK at ICU admission serves as a biomarker of severity in pH1N1 infection and may provide clinical insight into which patients are at higher risk of renal and respiratory artificial support, and require increased healthcare resources.
Abbreviations
- CK:
-
Creatine kinase
- LOS:
-
Length of stay
- MV:
-
Mechanical ventilation
- RRT:
-
Requirement of renal replacement techniques
- SARI:
-
Severe acute respiratory insufficiency
- AKI:
-
Acute kidney injury
- GTEI-SEMICYUC:
-
Infectious Disease Working Group of the Spanish Society of Intensive Care Medicine
- WHO:
-
World Health Organization
- RT-PCR:
-
Real-time polymerase chain reaction
- CDC:
-
Centers for Disease Control and Prevention
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- HIV:
-
Human immunodeficiency virus
- AIDS:
-
Acquired immune-deficiency syndrome
- SOFA:
-
Sequential Organ Failure Assessment
- CVVHF:
-
Continuous veno-venous hemofiltration
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA, Canadian critical care trials group H1N1 collaborative (2009) Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302:1872–1879
Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA (2009) Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 361:674–679
Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, Gadd EM, Lim WS, Semple MG, Read RC, Taylor BL, Brett SJ, McMenamin J, Enstone JE, Armstrong C, Nicholson KG, Influenza Clinical Information Network (FLU-CIN) (2010) Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–September 2009). Thorax 65:645–651
Lee N, Chan PK, Lui GC, Wong BC, Sin WW, Choi KW, Wong RY, Lee EL, Yeung AC, Ngai KL, Chan MC, Lai RW, Yu AW, Hui DS (2011) Complications and outcomes of pandemic 2009 Influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis 203:1739–1747
Viasus D, Paño-Pardo JR, Pachón J, Riera M, López-Medrano F, Payeras A, Fariñas MC, Moreno A, Rodríguez-Baño J, Oteo JA, Martínez-Montauti J, Torre-Cisneros J, Segura F, Gudiol F, Carratalà J; Novel Influenza A(H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI) (2011) Pneumonia complicating pandemic (H1N1) 2009: risk factors, clinical features, and outcomes. Medicine (Baltimore) 90:328–336
Viasus D, Paño-Pardo JR, Pachón J, Campins A, López-Medrano F, Villoslada A, Fariñas MC, Moreno A, Rodríguez-Baño J, Oteo JA, Martínez-Montauti J, Torre-Cisneros J, Segura F, Gudiol F, Carratalà J; Novel Influenza A (H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI) (2011) Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect 17:738–746
Martin-Loeches I, Papiol E, Rodríguez A, Díaz E, Zaragoza R, Granada RM, Socias L, Bonastre J, Valverdú M, Pozo JC, Luque P, Juliá-Narvaéz JA, Cordero L, Albaya A, Serón D, Rello J, The H1N1 SEMICYUC Working Group (2011) Acute kidney injury in critical ill patients affected by influenza A (H1N1) virus infection. Crit Care 15:R66
Tanaka T, Takada T, Takagi D, Takevama N, Kitazawa Y (1989) Acute renal failure due to rhabdomyolysis associated with echovirus 9 infection: a case report and review of literature. Jpn J Med 28:237–242
Singh U, Scheld WM (1996) Infectious etiologies of rhabdomyolysis: three case reports and review. Clin Infect Dis 22:642–689
Kumar K, Guirgis M, Zieroth S, Lo E, Menkis AH, Arora RC, Freed DH (2011) Influenza myocarditis and myositis: case presentation and review of the literature. Can J Cardiol 27:514–522
World Health Organization (2009) Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses. http://www.who.int/csr/resources/publications/SwineInfluenza_infectioncontrol.pdf. Accesed 29 April 2011
Centers for Disease Control and Prevention (2009) Interim Recommendations for Clinical Use of Influenza Diagnostic Tests During the 2009–2010 Influenza Season. http://www.cdc.gov/h1n1flu/guidance/diagnostic_tests.htm. Accesed 30 Sept 2009
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On be-half of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J (2008) Appendix: Table 2: clinical chemistry and immunology. In: Harrison’s principles of internal medicine, 17th edn. McGraw Hill Medical, New York, pp 8.738
Wallach J (2000) Introduction to normal values (reference ranges). In: Interpretation of diagnostic test, 7th edn. Lippincott Williams & Wilkins, Philadelphia, p 13
Brown SM, Jones BE, Jephson AR, Dean NC, Infectious Disease Society of America/American Thoracic Society (2009) Validation of the infectious disease society of america/american thoracic society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med 37:3010–3016
Ronco C, Levin A, Warnock DG, Mehta R, Kellum JA, Shah S, Molitoris BA, AKIN Working Group (2007) Improving outcomes from acute kidney injury (AKI): report on an initiative. Int J Artif Organs 30:373–376
Pérez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, Ramírez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Mondragon E, Cordova-Villalobos JA, INER Working Group on Influenza (2009) Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 361:680–689
Parikh M, Dolson G, Ramanathan V, Sangsiraprapha W (2010) Novel H1N1-associated rhabdomyolysis leading to acute renal failure. Clin Microbiol Infect 16:330–332
Fearnley RA, Lines SW, Lewington AJ, Bodenham AR (2011) Influenza A-induced rhabdomyolysis and acute kidney injury complicated by posterior reversible encephalopathy syndrome. Anaesthesia 66:738–742. doi:10.1111/j.1365-2044.2011.06752.x
Ayala E, Kagawa FT, Wehner JH, Tam J, Upadhyay D (2009) Rhabdomyolysis associated with 2009 influenza A(H1N1). JAMA 17:1863–1864
Shiuan-Chih Chen SC, Liu KS, Chang HR, Lee YT, Chen CC, Lee MC (2010) Rhabdomyolysis following pandemic influenza A (H1N1) infection. Neth J Med 68:317–319
Slobogean BL, Reilly CW, Alvarez CM (2011) Recurrent viral-induced compartment syndrome. Pediatr Emerg Care 27:660–662
Tosun MS, Ertekin V, Orbak Z (2012) Rhabdomyolysis-induced acute renal failure associated with 2009 influenza A (H1N1) virus infection in a child with Crigler-Najjar syndrome. J Emerg Med 42:310–311
Ayala E, Kagawa F, Tam J (2009) Rhabdomyolysis associated with 2009 influenza A (H1N1). JAMA 302:1863–1864
D’Silva D, Hewagama S, Doherty R, Korman TM, Buttery J (2009) Melting muscles: novel H1N1 influenza A associated rhabdomyolysis. Pediatr Infect Dis J 28:1138–1139
Gutiérrez RL, Ellis MW, Decker CF (2010) Rhabdomyolysis and pandemic (H1N1) 2009 pneumonia in adult. Emerg Infec Dis 16:565. doi:10.3201/eid1603.091818565
Lai CC, Wang CY, Lin HI (2010) Rhabdomyolysis and acute kidney injury associated with 2009 pandemic influenza A (H1N1). Am J Kidney Dis 55:615. doi:10.1053/j.ajkd.2010.01.002
Paredes R, Verbin S (2008) Rhabdomyolysis a review of clinical presentation, etiology, diagnosis, and management. Pediatr Emerg Care 24:262–268
Cervellin G, Comelli I, Lippi G (2010) Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 48:749–756
Khan FY (2009) Rhabdomyolysis: a review of the literature. Neth J Med 67:272–283
Miller M (2011) Clinical manifestations, diagnosis, and causes of rhabdomyolysis. UptoDate. http://www.uptodate.com/contents/clinical-manifestations-diagnosis-and-causes-of-rhabdomyolysis. Accessed 8 May 2011
Bosch X, Poch E, Grau JM (2009) Rhabdomyolysis and acute kidney injury. N Engl J Med 361:62–72
Acknowledgments
The authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article. Administrative support, logistics, and data storage were funded by resources assigned to node 18 (director: Jordi Rello) of CIBERES (Centro de Investigación Respiratoria en Red en Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain), being part of PCI Neumonía-CIBERES and the own research group. Data collection and database were conducted by Thiago Lisboa, Sandra Trefler, Mireia Llauradó, and Rosi Luque (salary funded by node 18 of CIBERES) and Alejandro Rodríguez (salary funded by Institut Català de la Salut).
Conflicts of interest
The authors do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
See Appendix for the pH1N1 GTEI/SEMICYUC investigators.
Appendix: pH1N1 participating investigators of the GTEI/SEMICYUC working group (coordinator: Rafael Zaragoza; associated coordinator: Alejandro Rodríguez)
Appendix: pH1N1 participating investigators of the GTEI/SEMICYUC working group (coordinator: Rafael Zaragoza; associated coordinator: Alejandro Rodríguez)
Andalucía
Pedro Cobo (Hospital Punta de Europa, Algeciras); Javier Martins (Hospital Santa Ana Motril, Granada); Cecilia Carbayo (Hospital Torrecardenas, Almería); Emilio Robles-Musso, Antonio Cárdenas, Javier Fierro (Hospital del Poniente, Almería); Dolores Ocaña Fernández (Hospital Huercal—Overa, Almería); Rafael Sierra (Hospital Puerta del Mar, Cádiz); Mª Jesús Huertos (Hospital Puerto Real, Cádiz); Juan Carlos Pozo, R. Guerrero (Hospital Reina Sofía, Córdoba); Enrique Márquez (Hospital Infanta Elena, Huelva); Manuel Rodríguez-Carvajal (Hospital Juan Ramón Jiménez, Huelva); Antonio Jareño, A. Estella (Hospital del SAS de Jerez, Jerez de la Frontera); José Pomares, José Luis Ballesteros (Hospital Universitario San Cecilio, Granada); Yolanda Fernández, Francisco Lobato, José F. Prieto, José Albofedo-Sánchez (Hospital Costa del Sol, Marbella); Pilar Martínez (Hospital Vírgen de la Victoria, Málaga); Miguel Ángel Díaz Castellanos (Hospital Santa Ana de Motril, Granada); Guillermo Sevilla (Clínica Sagrado Corazón, Sevilla); José Garnacho-Montero, Rafael Hinojosa, Esteban Fernández (Hospital Virgen del Rocío, Sevilla); Ana Loza, Cristóbal León (Hospital Universitario Nuestra Señora de Valme, Sevilla); Ángel Arenzana (Hospital Virgen de la Macarena, Sevilla); Dolores Ocaña (Hospital de la Inmaculada, Sevilla); Inés Navarrete (Hospital Virgen de las Nieves, Granada); Medhi Zaheri Beryanaki (Hospital de Antequera); Ignacio Sánchez (Hospital NISA Sevilla ALJARAFE, Sevilla).
Aragón
Manuel Luis Avellanas, Arantxa Lander, S. Garrido Ramírez de Arellano, M.I. Marquina Lacueva (Hospital San Jorge, Huesca); Pilar Luque (Hospital Lozano Blesa, Zaragoza); Ignacio González (Hospital Miquel Servet, Zaragoza); Jose Mª Montón (Hospital Obispo Polanco, Teruel); Paloma Dorado Regil (Hospital Royo Villanova, Zaragoza).
Asturias
Lisardo Iglesias, Carmen Pascual González (Hospital Universitario Central de Asturias—HUCA, Oviedo); J.M. Quiroga; Águeda García-Rodríguez (Hospital Valle del Nalón, Langreo).
Baleares
Lorenzo Socias, Pedro Ibánez, Marcío Borges-Sa, A. Socias, A. Del Castillo (Hospital Son Llàtzer, Palma de Mallorca); Ricard Jordà Marcos (Clínica Rotger, Palma de Mallorca); José M. Bonell (USP, Clínica Palmaplanas, Palma de Mallorca); Ignacio Amestarán (Hospital Son Dureta, Palma de Mallorca).
Canarias
Sergio Ruiz-Santana, Juan José Díaz, J. Ferrer, Jordi Sole-Violan (Hospital Dr. Negrín, Las Palmas de Gran Canaria); Sisón (Hospital Doctor José Molina, Lanzarote); David Hernández, Ana Trujillo, Luis Regalado (Hospital General la Palma, La Palma); Leonardo Lorente (Hospital Universitario de Canarias, Tenerife); Mar Martín (Hospital de la Candelaria, Tenerife), Sergio Martínez, J.J. Cáceres (Hospital Insular de Gran Canaria).
Cantabria
Borja Suberviola, P. Ugarte (Hospital Universitario Marqués de Valdecilla, Santander).
Castilla La Mancha
Fernando García-López (Hospital General, Albacete); Angel Álvaro Alonso, Antonio Pasilla (Hospital General La Mancha Centro, Alcázar de San Juan); Mª Luisa Gómez Grande (Hospital General de Ciudad Real, Ciudad Real); Antonio Albaya (Hospital Universitario de Guadalajara, Guadalajara); Alfonso Canabal, Luis Marina (Hospital Virgen de la Salud, Toledo); Almudena Simón (Hospital Nuestra Señora del Prado, Toledo); José María Añón (Hospital Virgen de la Luz, Cuenca).
Castilla y León
Juan B. López Messa (Complejo Asistencial de Palencia, Palencia); Mª Jesús López Pueyo (Hospital General Yagüe, Burgos); Zulema Ferreras (Hospital Universitario de Salamanca, Salamanca); Santiago Macias (Hospital General de Segovia, Segovia); José Ángel Berezo, Jesús Blanco Varela (Hospital Universitario Río Hortega, Valladolid); A. Andaluz Ojeda (Hospital Universitario, Valladolid); Antonio Álvarez Terrero (Hospital Virgen de la Concha, Zamora); Fabiola Tena Ezpeleta (Hospital Santa Bárbara, Soria); Zulema Paez, Álvaro García (Hospital Virgen Vega, Salamanca).
Catalunya
Rosa Mª Catalán (Hospital General de Vic, Vic); Miquel Ferrer, Antoni Torres (Hospital Clínic, Barcelona); Sandra Barbadillo (Hospital General de Catalunya – CAPIO, Barcelona); Lluís Cabré (Hospital de Barcelona, Barcelona); Assumpta Rovira (Hospital General de l’Hospitalet, L’Hospitalet); Francisco Álvarez-Lerma, Antonia Vázquez, Joan Nolla (Hospital Del Mar, Barcelona); Francisco Fernández, Joaquim Ramón Cervelló (Centro Médico Delfos, Barcelona); Rafael Mañéz, J. Ballús, Rosa Mª Granada (Hospital de Bellvitge, Barcelona); Jordi Vallés, Marta Ortíz, C. Guía (Hospital de Sabadell, Sabadell); Fernando Arméstar, Joaquim Páez (Hospital Dos De Mayo, Barcelona); Jordi Almirall, Xavier Balanzo (Hospital de Mataró, Mataró); Elena Arnau, Cesar Laborda, Jessica Souto, J.R. Masclans, Lluis Llopart, Ana Sanchez, Mercedes Palomar (Hospital Vall d’Hebron, Barcelona); Iñaki Catalán (Hospital Sant Joan de Déu, Manresa); Josep Mª Sirvent, Cristina Ferri, Nerea López de Arbina (Hospital Josep Trueta, Girona); Mariona Badía, Montserrat Valverdú-Vidal, Fernando Barcenilla (Hospital Arnau de Vilanova, Lleida); Mònica Magret (Hospital Sant Joan de Reus, Reus); M.F. Esteban, José Luna (Hospital Verge de la Cinta, Tortosa); Juan Mª Nava, J. González de Molina (Hospital Universitario Mutua de Terrassa, Terrassa); Zoran Josic (Hospital de Igualada, Igualada); Francisco Gurri (Hospital Quirón, Barcelona, Jordi Rello, Alejandro Rodríguez, Thiago Lisboa, Diego de Mendoza, Ana Parra, Evelyn Garcia (Hospital Universitario Joan XXIII, Tarragona); Rosa María Díaz (Hospital San Camil. Sant Pere de Ribes, Barcelona); Eduard Mesalles (Hospital Germans Trias i Pujol, Badalona).
Extremadura
Juliá-Narváez José (Hospital Infanta Cristina, Badajóz); Alberto Fernández-Zapata, Teresa Recio, Abilio Arrascaeta, Mª José García-Ramos, Elena Gallego (Hospital San Pedro de Alcántara, Cáceres); Fernándo Bueno (Hospital Virgen del Puerto, Plasencia); Mercedes Díaz (Hospital de Mérida, Mérida).
Galicia
Mª Lourdes Cordero, José A. Pastor, Luis Álvarez—Rocha (CHUAC, A Coruña); Dolores Vila (Hospital Do Meixoeiro, Vigo); Ana Díaz Lamas (Hospital Arquitecto Marcide, Ferrol); Javier Blanco Pérez, M. Ortiz Piquer (Hospital Xeral—Calde, Lugo); Eleuterio Merayo, Victor Jose López-Ciudad, Juan Cortez, Eva Vilaboy (Complejo Hospitalario de Ourense, Ourense); Eva María Saborido (Hospital Montecelo, Pontevedra); Raul José González (H. Miguel Domínguez, Pontevedra); Santiago Freita (Complejo Hospitalario de Pontevedra, Pontevedra); Ana María López; Julio Canabal, Enrique Ferres (Clinica Universitaria Santiago de Compostela, Santiago).
La Rioja
José Luis Monzón, Félix Goñi (Hospital San Pedro, Logroño).
Madrid
Frutos Del Nogal Sáez, M. Blasco Navalpotro (Hospital Severo Ochoa, Madrid); Mª Carmen García-Torrejón (Hospital Infanta Elena, Madrid); César Pérez–Calvo, Diego López (Fundación Jiménez Díaz, Madrid); Luis Arnaiz, S. Sánchez-Alonso, Carlos Velayos (Hospital Fuenlabrada, Madrid); Francisco del Río, Miguel Ángel González (Hospital Clínico San Carlos, Madrid); María Cruz Martín, José Mª Molina (Hospital Nuestra Señora de América, Madrid); Juan Carlos Montejo, Mercedes Catalán (Hospital Universitario 12 de Octubre, Madrid); Patricia Albert, Ana de Pablo (Hospital del Sureste, Arganda del rey); José Eugenio Guerrero, Jaime Benitez Peyrat (Hospital Gregorio Marañón, Madrid); Enrique Cerdá, Manuel Alvarez, Carlos Pey (Hospital Infanta Cristina, Madrid); Montse Rodríguez, Eduardo Palencia (Hospital Infanta Leonor, Madrid); Rafael Caballero (Hospital de San Rafael, Madrid); Concepción Vaquero, Francisco Mariscal, Susana García-Plaza (Hospital Infanta Sofía, Madrid); Nieves Carrasco (Hospital Universitario La Princesa, Madrid); Isidro Prieto, A. Liétor, R. Ramos (Hospital Ramón y Cajal, Madrid); Beatríz Galván, Juan C. Figueira, M. Cruz Soriano (Hospital La Paz, Madrid); P. Galdós, Bárbara Balandin Moreno (Hospital Puerta de Hierro, Madrid); Fernández del Cabo (Hospital Monte Príncipe, Madrid); Cecilia Hermosa, Federico Gordo (Hospital de Henares, Madrid); Alejandro Algora (Hospital Universitario Fundación Alcorcón, Madrid); Amparo Paredes (Hospital Sur de Alcorcón, Madrid); J.A. Cambronero (Hospital Universitario Príncipe de Asturias, Madrid); Sonia Gómez-Rosado (Hospital de Móstoles, Madrid); Luis Miguel Prado López (Hospital Sanitas La Zarzuela, Madrid).
Murcia
Sofía Martínez (Hospital Santa María del Rosell, Murcia); F. Felices Abad (Hospital Universitario Reina Sofía, Murcia); Mariano Martínez (Hospital Universitario Virgen de la Arrixaca, Murcia); Sergio Manuel Butí, Bernardo Gil Rueda, Francisco García (Hospital Morales Messeguer, Murcia).
Navarra
Enrique Maraví-Poma, I. Jiménez Urra, L. Macaya Redin, A. Tellería (Hospital Virgen del Camino, Pamplona); Josu Insansti (Hospital de Navarra, Pamplona).
País Vasco
Nagore González, Pilar Marco, Loreto Vidaur, Emilio Pérez-Trallero (Hospital de Donostia, San Sebastián); B. Santamaría (Hospital de Basurto, Bilbao); Juan Carlos Vergara, José Ramón (Hospital de Cruces, Bilbao); Alberto Manzano (Hospital Santiago Apóstol, Vitoria); Carlos Castillo Arenal (Hospital Txagorritxu, Vitoria); Pedro María Olaechea (Hospital Galdakano-Usansolo, Vizcaya).
Valencia
José Blanquer (Hospital Clinic Universitari, Valencia); Roberto Reig Valero, A. Belenger, Susana Altaba (Hospital General de Castellón, Castellón); Bernabé Álvarez-Sánchez (Hospital General de Alicante, Alicante); Santiago Alberto Picos (Hospital Torrevieja Salud, Alicante); Ángel Sánchez-Miralles (Hospital San Juan, Alicante); Juan Bonastre, M. Palamo, Javier Cebrian, José Cuñat (Hospital La Fe, Valencia); Belén Romero (Hospital de Manises, Valencia); Rafael Zaragoza (Hospital Dr. Peset, Valencia); Virgilio Paricio (Hospital de Requena, Valencia); Asunción Marques, S. Sánchez-Morcillo, S. Tormo (Hospital de la Ribera, Valencia). J. Latour (H.G. Universitario de Elche, Valencia), M. Ángel García (Hospital de Sagunto, Castellón).
Andorra
Antoli Ribas (Hospital Nuestra Señora de Meritxell, Andorra).
Rights and permissions
About this article
Cite this article
Borgatta, B., Pérez, M., Vidaur, L. et al. Elevation of creatine kinase is associated with worse outcomes in 2009 pH1N1 influenza A infection. Intensive Care Med 38, 1152–1161 (2012). https://doi.org/10.1007/s00134-012-2565-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2565-5