Abstract
Purpose
To compare the effects of two humidifier systems on endotracheal tube (ETT) resistance during mechanical ventilation, either an active heated humidifier (HH) or a passive heat and moisture exchanger (HME) was selected using current clinical recommendations.
Methods
This was a prospective clinical cohort study performed in an intensive care unit. Gas conditioning was performed using the HH in 22 patients and the HME in another 22. Patients were matched for endotracheal tube diameter, days of mechanical ventilation, simplified acute physiology score II (SAPS II), and fluid balance.
Results
Used-ETT resistance was measured immediately after extubation. Unused-ETT resistance was calculated with an identical, clean ETT. No differences were found between the HH and HME groups in ETT diameter (7.9 ± 0.4 vs. 7.9 ± 0.3 mm; p = 0.98), days of mechanical ventilation (11.3 ± 7.7 vs. 9.5 ± 4.5; p = 0.34), SAPS II (41.0 ± 13.6 vs. 42.0 ± 11.7; p = 0.79), or fluid balance (−2,552 ± 6,268 vs. −2,579 ± 5,422 mL; p = 0.98). ETT resistance increased from intubation to extubation: from 6.8 ± 1.1 to 10.6 ± 4.3 cmH2O L−1 s−1 in the HH group, (p < 0.001) and from 6.8 ± 1.1 to 10.2 ± 3.8 cmH2O L−1 s−1 in the HME group (p < 0.001), which is a 53% average increase in resistive load.
Conclusions
We did not find differences between the two types of humidifiers in terms of airflow resistance during prolonged mechanical ventilation when the devices were selected on the basis of individual clinical needs. The increase in resistive load is physiologically relevant.
Similar content being viewed by others
Introduction
Humidification and warming of inspired gases are of major importance during mechanical ventilation because the upper respiratory airway is bypassed by the endotracheal tube [1]. Two types of humidifiers are commonly used in clinical practice: heat and moisture exchangers (HME) and heated humidifiers (HH). Insufficient or excessive humidification can provoke airway mucosa dysfunction and abnormal humidification of respiratory secretions, eventually leading to endotracheal tube occlusion [2, 3]. The type of humidification, selected for use in this setting, is relevant because devices can exhibit disparate humidification efficiency [4, 5].
While patients are intubated and mechanically ventilated, respiratory secretions adhere to the inner surface of the endotracheal tube (ETT). As the diameter of the tube decreases, airflow resistance increases. This effect is common in acute respiratory failure patients under mechanical ventilation [6, 7] and it may be greater with prolonged use of passive humidification devices than with active devices [8, 9]. ETT patency has been proposed to indirectly reflect the quality of inspired gas humidification during mechanical ventilation [8]. Current data show no preferential performance of either HME or HH devices in mechanically ventilated patients concerning the incidence of ventilator-associated pneumonia, mortality, or morbidity [10, 11]. The type of humidification used during routine mechanical ventilation should be chosen on the basis of a patient’s underlying disease [12, 13], respiratory mechanics [14], quality of respiratory secretions [3, 15], the mechanical ventilation settings [16], and ambient and patient temperature [17, 18].
ETT narrowing is associated with increased work of breathing. It can prolong weaning from mechanical ventilation and artifactually alter the breathing pattern [14, 19, 20]. Two single-center studies observed that ETT resistance was significantly higher when inspiratory gases were conditioned via an HME as compared to an HH [8, 9]. These data, however, have not been replicated. The aim of our study was to assess the impact of two humidification devices (passive HME vs. active HH) on in vivo ETT resistance to airflow during long-term invasive mechanical ventilation in unselected acute respiratory failure patients. Preliminary data from this study have been previously presented [21].
Materials and methods
The study was performed in the Intensive Care Unit at Hospital de la Santa Creu i Sant Pau, Barcelona (Spain). Given the nature of measurements and the fact that HH and HME are used in routine practice during mechanical ventilation in our institution, the requirement for signed informed consent was waived by the institutional ethics committee after approval of the protocol. All patients included in the study were intubated and mechanically ventilated for more than 48 h. Exclusion criteria were age below 18, tracheotomy, or enrolment in another clinical trial.
The mechanical ventilation parameters used in the study were those used in routine clinical management. ETTs used were high-volume, low-pressure cuffed tubes (Rüschelit® Rüsch; Karmunting, Malaysia). They were all the same brand and model, but internal diameters differed according to patients’ clinical and anthropometric characteristic. Ventilator circuits remained unchanged throughout the course of mechanical ventilation. Secretion removal from ETTs or upper airways was conducted following open suction technique based on clinical detection or suspicion of secretions due to increased airway pressures in the ventilator display. Only the first episode of extubation was included in the study in patients who presented multiple episodes of extubation. Our routine clinical practice considers using an HH based on current recommendations [18, 22] in the following scenarios: previous presence of profuse bloody or copious mucous secretions, tenacious sputum, episode of ETT or tracheotomy occlusion, acute respiratory distress syndrome, asthma, exacerbation of chronic obstructive disease, and hypothermia.
Each ETT from a patient humidified with an HH was matched with an ETT from a patient in whom an HME was used. Four variables were assessed, in the following order: ETT diameter, days of mechanical ventilation, simplified acute physiology score II (SAPS II), and fluid balance.
The HH devices (Fisher & Paykel; MR 290 and MR 850 ALU; Panmure, New Zealand) were placed in the inspiratory limb of the circuit in accordance with the manufacturer’s recommendations. The HME devices (Edit Flex, Datex Engstrom®, Helsinki, Finland) had a dead space of 90 mL that included an integrated flexible tube and a filter. The “in vitro” HME resistive pressure drop described by the manufacturer was 1.4 cmH2O at a constant flow of 60 L/min. HMEs were placed between the Y piece and the ETT. Mechanical ventilation was maintained at routine parameters established by the responsible physician.
ETT resistance was measured directly from the respiratory monitoring system of the Puritan-Bennett 7200 ventilator (Puritan-Bennett Corporation, Carlsbad, CA, USA) after proper calibration of the ventilator following the manufacturer’s recommendation and using a standard disposable breathing circuit connected to the proximal end of the ETT in an identical manner as done with patients. The distal end of the ETT was open to the atmosphere. ETT resistance (R measured in cmH2O L−1 s−1) was calculated as the pressure drop between the proximal and distal end of the ETT (measured in cmH2O) divided by flow (measured in L/s). During ETT resistance measurements, flow was passed constantly at 60 L/min (1 L/s). For clean ETT resistance (“unused-ETT”) and immediately after extubation ETT resistance measurements (“used-ETT”), we used the same setup. Experimental setting and measurements are shown in the Electronic Supplementary Material (ESM Figs. 1, 2).
We calculated the theoretical increase in work of breathing (WOB), expressed in joules (J/min), provoked by the increased resistance of the ETT after use (i.e., the difference between after extubation ETT resistance and unused-ETT resistance). This theoretical calculation was performed assuming a respiratory rate of 20/min, inspiratory time of 0.5 s, and constant inspiratory flow of 60 L/min (i.e., a breathing pattern with a respiratory rate of 20/min, a tidal volume of 500 mL, inspiratory time of 0.5 s, and an expiratory time of 2.5 s).
All patients in the study were subjected to our usual weaning process. This was accomplished by reducing the pressure support ventilation (PSV) and positive end-expiratory pressure (PEEP) levels as described [23, 24]. Planned extubation was performed when a patient tolerated low levels of PSV (≈7 cmH2O) without PEEP or T-piece trial with FiO2 less than 0.5, between 30 and 120 min. We performed endotracheal suctioning during the extubation procedure. This suctioning consisted of disconnecting the patient from the ventilator and inserting a suction catheter of 12–14 Fr through the ETT into the airways until resistance was met. The catheter was then pulled back 1–2 cm. Negative suctioning pressure at 150–200 mmHg was continuously applied for 10–15 s while the catheter was rotated and removed simultaneously with the ETT [25, 26]. Patients not subjected to weaning but eventually extubated (i.e., those who died) were also included.
Statistical analysis
Sample size calculation was performed assuming the following: (1) we calculated the baseline resistance of unused-ETT, which was of 6.8 ± 1.1 cmH2O L−1 s−1; (2) a difference in ETT resistance of 1.0 cmH2O L−1 s−1 between the two humidification systems would be considered as clinically relevant according to previously published data [8, 9]; (3) taking into consideration (1) and (2), to detect a two-tailed significant difference (type I error of 5%; α = 0.05) with at least an 80% statistical power (β = 0.20), 22 patients per group are required; (4) to confirm normal data distribution and equal variances between groups we performed the Kolmogorov–Smirnov test and the Levene test, respectively. Therefore, since the data regarding used-ETT resistances were normally distributed and variances did not show differences, we used the Student’s t test for the comparison. For non-normally distributed variables we used the Mann–Whitney U test. Dichotomous variables were compared using the chi-square method with a two-tailed Fisher’s exact test. A p value less than 0.05 was considered statistically significant. Data are expressed as means ± standard deviation (SD). The SPSS® (version 17.0, Chicago, IL, USA) statistical software was used for statistical analysis.
Results
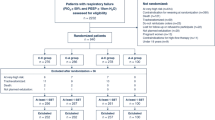
The study involved a total of 44 ETT that were recovered from prospective consecutive matched patients. Twenty-two ETTs were from patients humidified with an HH (HH group) and 22 were from patients humidified with an HME (HME group). Table 1 lists patients’ demographic data, the main indication for mechanical ventilation, and clinical characteristics at admission. In addition, Table 1 shows number of days intubated, days of bronchodilator therapy, fluid balance, ETT diameter, and unused-ETT and used-ETT flow resistance.
All patients were orotracheally intubated and ETT diameters ranged from 7.0 to 8.5 mm. The measured ETT resistances were statistically higher among the endotracheal tubes after extubation than in the ETT before use (10.4 ± 4.0 vs. 6.8 ± 1.1 cmH2O L−1 s−1; p < 0.001). Figure 1 shows patient per patient ETT resistance changes in the HH and HME groups. ETT resistances increased significantly in both groups: from 6.8 ± 1.1 to 10.6 ± 4.3 cmH2O L−1 s−1 (p < 0.001) in HH patients and from 6.8 ± 1.1 to 10.2 ± 3.8 cmH2O L−1 s−1 (p < 0.001) in HME patients. Increases in ETT resistance for endotracheal tubes did not correlate with mechanical ventilation duration in the HH group (r = 0.067; p = 0.766) or in the HME group (r = 0.117; p = 0.603). The average increase of ETT resistances between groups was similar (57 vs. 51%, p = 0.771; HH and HME, respectively). Besides, Fig. 1 shows the distribution of the diameter of ETTs at baseline and the theoretical effective inner diameter of ETTs related to changes in the resistance measured after extubation.
Black squares show flow resistance of unused-ETTs in relation to their diameter. Circles show individual values in ETT resistance measured after extubation, from patients humidified with HH (red circles) and from patients humidified with HME (blue circles). All resistance values were calculated at a constant flow of 1 L/s. All ETTs used were from the same brand and model varying only in the internal diameter among them. The dotted line represents the exponential increase in flow resistance in relation to the diameter of the unused-ETTs. As can be seen, 15 (34%) of used-ETTs had real resistances at extubation corresponding to unused-ETT with inner diameter below 7 mm
As expected, the estimated WOB related to the resistive pressure drop across the ETT was significantly higher in the used-ETT compared with the unused-ETT: 10.4 ± 4.0 vs. 6.8 ± 1.1 J/min, (p < 0.001). This estimate assumes a tidal volume of 500 mL, a constant inspiratory flow of 1 L/s, and a respiratory rate of 20/min.
Fourteen (32%) patients developed ventilator-associated pneumonia (VAP) (7 in the HH group and 7 in the HME group; p = 1). The duration of mechanical ventilation was significantly longer for the 14 patients that presented VAP than in patients who did not suffer VAP (14.6 ± 6.7 vs. 8.4 ± 5.1 days; p = 0.002). Nevertheless, ETT resistance in endotracheal tubes removed from patients who developed VAP showed no statistical differences compared with ETTs removed from patients without VAP (9.6 ± 3.3 vs. 10.1 ± 4.3 cmH2O L−1 s−1 respectively; p = 0.33).
Five patients in the HH group (23%) and three in the HME group (14%) required reintubation for respiratory failure after extubation (p = 0.69). No differences in intensive care unit (ICU) mortality were seen between the two groups: 4/22 (18%) patients died in each group.
Discussion
The main finding in this study was that we did not observe any differences between HH and HME in endotracheal tube resistance as used in routine clinical practice. ETT resistance increased significantly from intubation to extubation day, representing a non-negligible increase in mechanical workload with both devices.
Humidification devices decrease the adherence of respiratory secretion onto the ETT surface by conditioning the inspired gases [22]. Nevertheless, their efficiency regarding humidification levels [27, 28] and incidence of ETT occlusion [8] is highly variable. Our results confirm that deposits of respiratory secretions on the inner wall of ETTs reduce their lumen and thus increase airflow resistance.
In contrast with findings by Villafane et al. [8] and Jaber et al. [9], we did not observe differences in resistance between patients using HHs and patients using HMEs. These authors [8, 9] studied patients who were randomly assigned to different types of airway humidifiers, whereas we performed a matched-pair study controlled for ETT diameter, days of mechanical ventilation, SAPS II, and fluid balance. Our patients were assigned to HH or HME according to clinical needs and common recommendations [3, 12–15]. This clinical selection of the type of humidifier carried out in our study, according to the main respiratory diagnosis and secretion management needs, instead of randomization performed in those studies, may explain the differences found in the ETT resistance over time when comparing our study with those previously published. The fact that the HH and HME populations were not strictly identical may suggest a clinical bias favoring the use of one system over the other. This is, however, difficult to ascertain since in our study we did not analyze either quantity or quality of respiratory secretions.
An increase in mechanical workload due to a reduction in ETT inner diameter may generate a spurious weaning trial failure and prolong mechanical ventilation [14, 19, 20, 29, 30]. This deleterious situation can be avoided by applying adequate inspiratory pressure support [19, 31], but titration of pressure support is almost impossible to predict individually in these circumstances. Furthermore, it must be taken into account that HMEs may increase inspiratory resistance, dead space ventilation, inspiratory work of breathing, and dynamic hyperinflation. These variables may pose an extra burden on the respiratory muscles during the weaning process, leading to clinical intolerance to spontaneous breathing trials [14, 16, 32].
In a study involving healthy volunteers, Shapiro et al. [29] described that increases in WOB were magnified when ETT diameter decreased below 7 mm. The increase in WOB caused by the increase in resistance of ETTs may be clinically relevant in certain patients. In our study no patients were intubated with an ETT smaller than 7 mm. Nevertheless, we showed that in 34% of our patients (7 in the HH group and 8 in the HME group) the ETT airflow resistance at extubation time corresponded to the resistance of a clean ETT with an inner diameter between 5.5 and 7 mm (Fig. 1).
Many studies have proposed ETT patency as an indirect parameter to reflect gas conditioning during mechanical ventilation [7–9, 33–35]. Several of these studies [7–9] did not find differences between HMEs and HHs in the overall reduction in ETT mean diameter in the short term (5–6 days). Nevertheless, Shah et al. [7] and Jaber et al. [9] described significantly higher ETT airflow resistance with HMEs than with HHs in prolonged mechanical ventilation (10 ± 6 days). However, over a virtually identical duration of mechanical ventilation, we did not find statistical differences between the two devices in ETT airflow resistance. This could be explained as a result of differences between studies in clinical strategies, concerning, for example, the efficiency of humidifiers, patient selection, mechanical ventilation strategy, diagnosis and management of VAP, and strategies for suctioning airway secretions. ETT resistance may also be influenced by general patient hydration [6, 36] but we did not find statistically significant differences in fluid balance in our two groups. Besides, as reported by Boqué and co-workers [6], we did not find a correlation between the increase in ETT resistance and the days of ETT use.
To avoid the influence of patients’ spontaneous activity and flow pattern on the measurements of airflow resistance we studied isolated ETT resistance [36, 37]. We measured the resistance for each ETT immediately post-extubation maintaining identical ETT deformation and secretion accumulation and trying to minimize any change of the mucous material adhered within the ETT [8, 38]. Theoretically, our routine ETT suctioning procedure may cause artifacts in the results of the study because of mucus secretion removal from the ETT during extubation and measurement of resistance of the used-ETT after it. However, this practice was always identical in the HH and HME groups and we thus believe that the impact on results is minimal since our practice was equally distributed between the two groups.
With respect to patients who develop VAP, the reduction in the internal diameter of an ETT due to increases of respiratory secretions has been proposed as a factor that increases mechanical ventilation days [7]. Our data did not show higher ETT resistance in patients with VAP compared to that in patients without VAP.
The present study has several limitations. First, this was a case–control study with a sample size of 22 patients per group in which we did not randomly select the humidification device type. Second, as diagnoses at inclusion differed between groups and we did not measure heating or humidification levels we can not guarantee that the degree of humidification was identical. Furthermore, although we indirectly assessed gas conditioning (ETT airflow resistance) with both humidification devices, we did not examine secretion characteristics or eventual epithelial respiratory damage. Third, our theoretical increases in WOB probably overestimate the real workload attributable to increased resistance of the ETT, because we did the calculation assuming a gas flow of 1 L/s. Actually, Vassilakopoulos et al. [39] measured a mean inspiratory flow of 0.71 ± 0.19 L/s with an average tidal volume of 460 mL and average respiratory rate of 27/min during a successful T-piece trial.
In conclusion we did not find differences between the two types of humidifiers in terms of airflow resistance. However, ETT resistance increased significantly during mechanical ventilation with both the HH and HME devices. Such increases in resistance may be relevant from a clinical point of view, in particular, during the weaning phase from mechanical ventilation and the performance of spontaneous breathing trials.
References
Chalon J, Patel C, Ali M, Ramanathan S, Capan L, Tang CK, Turndorf H (1979) Humidity and the anesthetized patient. Anesthesiology 50:195–198
Williams R, Rankin N, Smith T, Galler D, Seakins P (1996) Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med 24:1920–1929
Kapadia FN (2001) Factors associated with blocked tracheal tubes. Intensive Care Med 27:1679–1681
Lellouche F, Taille S, Maggiore SM, Qader S, L’Her E, Deye N et al (2004) Influence of ambient and ventilator output temperatures on performance of heated-wire humidifiers. Am J Respir Crit Care Med 170:1073–1079
Lellouche F, Taille S, Lefrancois F, Deye N, Maggiore SM, Jouvet P, Ricard JD, Fumagalli B, Brochard L (2009) Humidification performance of 48 passive airway humidifiers: comparison with manufacturer data. Chest 135:276–286
Boqué MC, Gualis B, Sandiumenge A, Rello J (2004) Endotracheal tube intraluminal diameter narrowing after mechanical ventilation: use of acoustic reflectometry. Intensive Care Med 30:2204–2209
Shah C, Kollef MH (2004) Endotracheal tube intraluminal volume loss among mechanically ventilated patients. Crit Care Med 32:120–125
Villafane MC, Cinnella G, Lofaso F, Isabey D, Harf A, Lemaire F, Brochard L (1996) Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesiology 85:1341–1349
Jaber S, Pigeot J, Fodil R, Maggiore S, Harf A, Isabey D, Brochard L, Louis B (2004) Long-term effects of different humidification systems on endotracheal tube patency: evaluation by the acoustic reflection method. Anesthesiology 100:782–788
Lacherade JC, Auburtin M, Cerf C, Van de Louw A, Soufir L, Rebufat Y, Rezaiguia S, Ricard JD, Lellouche F, Brun-Buisson C, Brochard L (2005) Impact of humidification systems on ventilator-associated pneumonia: a randomized multicenter trial. Am J Respir Crit Care Med 172:1276–1282
Siempos II, Vardakas KZ, Kopterides P, Falagas ME (2007) Impact of passive humidification on clinical outcomes of mechanically ventilated patients: a meta-analysis of randomized controlled trials. Crit Care Med 35:2843–2851
Prin S, Chergui K, Augarde R, Page B, Jardin F, Vieillard-Baron A (2002) Ability and safety of a heated humidifier to control hypercapnic acidosis in severe ARDS. Intensive Care Med 28:1756–1760
Morán I, Bellapart J, Vari A, Mancebo J (2006) Heat and moisture exchangers and heated humidifiers in acute lung injury/acute respiratory distress syndrome patients. Effects on respiratory mechanics and gas exchange. Intensive Care Med 32:524–531
Iotti GA, Olivei MC, Palo A, Galbusera C, Veronesi R, Comelli A, Brunner JX, Braschi A (1997) Unfavorable mechanical effects of heat and moisture exchangers in ventilated patients. Intensive Care Med 23:399–405
Kapadia FN, Bajan KB, Singh S, Mathew B, Nath A, Wadkar S (2001) Changing patterns of airway accidents in intubated ICU patients. Intensive Care Med 27:296–300
Girault C, Breton L, Richard JC, Tamion F, Vandelet P, Aboab J, Leroy J, Bonmarchand G (2003) Mechanical effects of airway humidification devices in difficult to wean patients. Crit Care Med 31:1306–1311
Lellouche F, Qader S, Taille S, Lyazidi A, Brochard L (2006) Under-humidification and over-humidification during moderate induced hypothermia with usual devices. Intensive Care Med 32:1014–1021
American Association for Respiratory Care (1992) AARC clinical practice guideline: humidification during mechanical ventilation. Respir Care 37:887–890
Brochard L, Rua F, Lorino H, Lemaire F, Harf A (1991) Inspiratory pressure support compensates for the additional work of breathing caused by the endotracheal tube. Anesthesiology 75:739–745
Rumbak MJ, Walsh FW, Anderson WM, Rolfe MW, Solomon DA (1999) Significant tracheal obstruction causing failure to wean in patients requiring prolonged mechanical ventilation: a forgotten complication of long-term mechanical ventilation. Chest 115:1092–1095
Cabello B, Morán I, Manero E, Manzanares E, Mancebo J (2007) Comparison of two humidificator systems on endotracheal tube resistance. Intensive Care Med 33(Suppl 2):A(532)
Branson RD (2006) Humidification of respired gases during mechanical ventilation: mechanical considerations. Respir Care Clin N Am 12:253–261
Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, Dojat M, Cabello B, Bouadma L, Rodriguez P, Maggiore S, Reynaert M, Mersmann S, Brochard L (2006) A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med 174:894–900
Cabello B, Thille AW, Roche-Campo F, Brochard L, Gomez FJ, Mancebo J (2010) Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med 36:1171–1179
Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, Richard JC, Mancebo J, Lemaire F, Brochard L (2003) Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med 167:1215–1224
Fernández MD, Piacentini E, Blanch L, Fernández R (2004) Changes in lung volume with three systems of endotracheal suctioning with and without pre-oxygenation in patients with mild-to-moderate lung failure. Intensive Care Med 30:2210–2215
Thomachot L, Viviand X, Arnaud S, Boisson C, Martin CD (1998) Comparing two heat and moisture exchangers, one hydrophobic and one hygroscopic, on humidifying efficacy and the rate of nosocomial pneumonia. Chest 114:1383–1389
Thomachot L, Leone M, Razzouk K, Antonini F, Vialet R, Martin C (2002) Randomized clinical trial of extended use of a hydrophobic condenser humidifier: 1 vs. 7 days. Crit Care Med 30:232–237
Shapiro M, Wilson RK, Casar G, Bloom K, Teague RB (1986) Work of breathing through different sized endotracheal tubes. Crit Care Med 14:1028–1031
Iotti GA, Olivei MC, Braschi A (1999) Mechanical effects of heat-moisture exchangers in ventilated patients. Crit Care 3:R77–82
Fiastro JF, Habib MP, Quan SF (1988) Pressure support compensation for inspiratory work due to endotracheal tubes and demand continuous positive airway pressure. Chest 93:499–505
Pelosi P, Solca M, Ravagnan I, Tubiolo D, Ferrario L, Gattinoni L (1996) Effects of heat and moisture exchangers on minute ventilation, ventilatory drive, and work of breathing during pressure-support ventilation in acute respiratory failure. Crit Care Med 24:1184–1188
Cohen IL, Weinberg PF, Fein IA, Rowinski GS (1988) Endotracheal tube occlusion associated with the use of heat and moisture exchangers in the intensive care unit. Crit Care Med 16:277–279
Roustan JP, Kienlen J, Aubas P, Aubas S, du Cailar J (1992) Comparison of hydrophobic heat and moisture exchangers with heated humidifier during prolonged mechanical ventilation. Intensive Care Med 18:97–100
Martin C, Papazian L, Perrin G, Bantz P, Gouin F (1992) Performance evaluation of three vaporizing humidifiers and two heat and moisture exchangers in patients with minute ventilation greater than 10 L/min. Chest 102:1347–1350
Wright PE, Marini JJ, Bernard GR (1989) In vitro versus in vivo comparison of endotracheal tube airflow resistance. Am Rev Respir Dis 140:10–16
Straus C, Louis B, Isabey D, Lemaire F, Harf A, Brochard L (1998) Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med 157:23–30
El-Khatib MF, Husari A, Jamaleddine GW, Ayoub CM, Bou-Khalil P (2008) Changes in resistances of endotracheal tubes with reductions in the cross-sectional area. Eur J Anaesthesiol 25:275–279
Vassilakopoulos T, Zakynthinos S, Roussos C (1998) The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med 158:378–385
Acknowledgment
B.C. was supported by grants from the Instituto de Salud Carlos III (Expedient CM04/00096, Ministerio de Sanidad) and the Instituto de Recerca Hospital de la Santa Creu i Sant Pau.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morán, I., Cabello, B., Manero, E. et al. Comparison of the effects of two humidifier systems on endotracheal tube resistance. Intensive Care Med 37, 1773–1779 (2011). https://doi.org/10.1007/s00134-011-2351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2351-9