Abstract
Purpose
Immunoparalysis defined by prolonged monocyte human leukocyte antigen DR depression is associated with adverse outcomes in adult severe sepsis and can be reversed with granulocyte macrophage colony-stimulating factor (GM-CSF). We hypothesized that immunoparalysis defined by whole-blood ex vivo lipopolysaccharide-induced tumor necrosis factor-alpha (TNFα) response <200 pg/mL beyond day 3 of multiple organ dysfunction syndrome (MODS) is similarly associated with nosocomial infection in children and can be reversed with GM-CSF.
Methods
In study period 1, we performed a multicenter cohort trial of transplant and nontransplant multiple organ dysfunction syndrome (MODS) patients (≥2 organ failure). In study period 2, we performed an open-label randomized trial of GM-CSF therapy for nonneutropenic, nontransplant, severe MODS patients (≥3 organ failure) with TNFα response <160 pg/mL.
Results
Immunoparalysis was observed in 34% of MODS patients (n = 70) and was associated with increased nosocomial infection (relative risk [RR] 3.3, 95% confidence interval [1.8–6.0] p < 0.05) and mortality (RR 5.8 [2.1–16] p < 0.05). TNFα response <200 pg/mL throughout 7 days after positive culture was associated with persistent nosocomial infection, whereas recovery above 200 pg/mL was associated with resolution of infection (p < 0.05). In study period 2, GM-CSF therapy facilitated rapid recovery of TNFα response to >200 pg/mL by 7 days (p < 0.05) and prevented nosocomial infection (no infections in seven patients versus eight infections in seven patients) (p < 0.05).
Conclusions
Similar to in adults, immunoparalysis is a potentially reversible risk factor for development of nosocomial infection in pediatric MODS. Whole-blood ex vivo TNFα response is a promising biomarker for monitoring this condition.
Similar content being viewed by others
Introduction
Critically ill children with a 2-week stay in the pediatric intensive care unit (PICU) have 50% risk for developing nosocomial sepsis [1]. While rates of nosocomial infection can be reduced through use of Centers for Disease Control and Prevention (CDC)-recommended preventive measures including strict hand-washing, aseptic techniques, and ventilator-associated pneumonia prevention bundles, this risk cannot be completely eliminated. Literature from the oncology, transplantation, and human immunodeficiency virus fields has shown that children with impaired immune function are at higher risk for development of nosocomial infection. Lymphopenia, neutropenia, and use of exogenous immunosuppression are associated with development of infectious complications in critically ill children [2–4].
The monocyte is an important innate immune cell in the initiation and regulation of the inflammatory response. Reduced expression of the monocyte cell surface molecule human leukocyte antigen (HLA)-DR, important for antigen presentation, is associated with adverse outcomes in adults, including development of nosocomial infection and death in sepsis [5, 6], trauma [7], transplantation [8], and pancreatitis [9]. Quantification of the capacity of whole blood to produce the proinflammatory cytokine tumor necrosis factor (TNF)-α after ex vivo stimulation with lipopolysaccharide (LPS) has been used as a second biomarker of monocyte function in critical illness, because LPS stimulation of the Toll-like receptor (TLR)-4 complex on monocytes should induce rapid TNFα transcription, translation, and release. Reduced capacity to produce TNFα after ex vivo stimulation with LPS is associated with adverse outcomes following adult sepsis [10] and trauma [11] as well as pediatric cardiopulmonary bypass [12].
The term “immunoparalysis” describes a clinical syndrome in which depressed monocyte biomarker levels are associated with adverse outcomes and increased nosocomial infection risk. Volk and colleagues have defined immunoparalysis in adults as prolonged monocyte HLA-DR expression below 30%, or reduction in whole-blood ex vivo LPS-induced TNFα response to <200 pg/mL for more than 5 days [10, 13]. They found that rapid tapering of immune-suppressant therapy in septic transplant patients with immunoparalysis increased HLA-DR expression to >30% and reduced mortality without causing organ rejection [8]. Most recently they reported that treatment of adults with severe sepsis with low-dose GM-CSF in a randomized trial resulted in reversal of immunoparalysis and attainment of beneficial clinical outcomes [14]. Interferon (IFN)γ or granulocyte macrophage colony-stimulating factor (GM-CSF) have been shown, in other small adult sepsis studies, both to increase monocyte HLA-DR expression and whole-blood ex vivo LPS responsiveness [10, 14–18] and to reduce infection.
In our present pediatric study, we tested the related hypotheses that immunoparalysis defined by whole-blood ex vivo LPS-stimulated TNFα response <200 pg/mL beyond day 3 commonly occurs in children with MODS and is associated with increased risk for developing secondary infection. We further tested the hypothesis that GM-CSF treatment can facilitate reversal of immunoparalysis and reduction of nosocomial infection.
Materials and methods
Patients-Institutional Review Board approval was obtained for this study at Children’s Hospital of Pittsburgh (Pittsburgh, PA) and Nationwide Children’s Hospital (Columbus, OH). Parental informed consent and patient assent, when appropriate, were obtained before enrollment.
Study 1
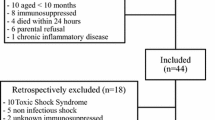
Patients were eligible for enrollment in the observational study if they fulfilled the following criteria: admission to the pediatric intensive care unit, dysfunction of two or more organs, and presence of an indwelling vascular catheter. Patients were excluded if aggressive therapy was not sought. Organ dysfunction was measured according to the Organ Failure Index (OFI, range 1–6) [19]. Blood samples were collected on days 3, 7, and 14 and weekly thereafter following the development of multiple organ dysfunction. The first 27 subjects in the cohort study underwent measurement of both monocyte HLA-DR expression and whole-blood ex vivo LPS-induced TNFα response. The remaining subjects underwent measurement of only TNFα response. Peripheral blood was obtained in Vacutainer tubes (BD Vacutainer, Franklin Lakes, NJ). Healthy control patients were recruited from the outpatient phlebotomy area and were sampled once. Children with fever within the past 24 h, or history of a chronic inflammatory disease, or those receiving antibiotics, or those with history of malignancy or transplantation were ineligible to serve as controls.
Study 2
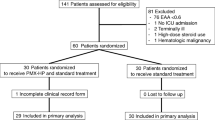
Nonneutropenic, nontransplant patients with dysfunction of ≥3 organs and with ex vivo LPS-induced TNFα response <160 pg/mL on day 3 of MODS were eligible for enrollment in the prospective, randomized, open-label GM-CSF study. These criteria were picked because patients with these characteristics in study 1 showed 100% risk of developing nosocomial infection. Patients were excluded if aggressive therapy was not sought. Parents were asked for consent to have their children tested for immunoparalysis. In case the child had TNFα response <160 pg/mL at 48 h they were then asked to consent to randomization to GM-CSF or standard therapy. Patients were randomized to GM-CSF 125 μg/m2 per day infused intravenously over a minimum of 12 h for 7 days, or to standard care. Randomization in blocks of four was blinded in the pharmacy. A white blood count threshold of >30,000 cells/mm3 was set as a criterion for early stoppage of GM-CSF treatment.
Whole-blood ex vivo LPS-stimulated TNFα response
Within 1 h of collection, 50 μl heparinized whole blood was mixed with 500 μl stimulation solution containing phenol-extracted LPS from Salmonella abortus equi (500 pg/mL) and incubated at 37°C for 4 h using a commercial ex vivo stimulation kit (Milenia Biotec, Bad Neuheim, Germany). After centrifugation for 5 min at 1,000 × g, the supernatant was stored at −80°C for subsequent TNFα measurement by chemiluminescence using the Immulite automated chemiluminometer (Diagnostic Products Corp., Los Angeles, CA; Siemens Medical Solutions Diagnostics, Deerfield, IL).
Monocyte HLA-DR expression
Whole blood, collected in ethylenediamine tetraacetic acid (EDTA) tubes, was stained with phycoerythrin (PE)-labeled anti-HLA-DR and fluorescein isothiocyanate (FITC)-labeled anti-CD14 antibodies (Becton–Dickinson, San Jose, CA) and prepared using the whole-blood lysis technique. After fixation with 2% paraformaldehyde, the percentage of HLA-DR+ cells among the CD14+ cell population was determined by flow cytometry.
Plasma interleukin (IL)-6 measurement
Within 1 h of collection, the supernatant from a centrifuged, fresh heparinized whole-blood specimen was collected and stored at −80°C for subsequent measurement by chemiluminescence using the Immulite automated chemiluminometer (Diagnostic Products Corp., Los Angeles, CA; Siemens Medical Solutions Diagnostics, Deerfield, IL).
Definitions
Immunoparalysis was defined by whole-blood ex vivo LPS-stimulated TNFα response <200 pg/mL after 3 days of multiple organ dysfunction or at death. Nosocomial infection was defined by CDC criteria as a new bacterial (Staphylococcus epidermidis excluded) or fungal infection after 48 h in a patient with fever, hypotension or oliguria. Mortality was defined as death during PICU stay. Resolved nosocomial infection was defined as absence of subsequent positive cultures during the next 7 days after an initial positive culture. Persistent nosocomial infection was defined as persistently positive cultures on more than 1 day during the next 7 days or proven infection at autopsy. Rapid tapering of immune suppression was defined as a drop of >50% in cyclosporine A or tacrolimus level within 72 h.
Clinical data collected included age, diagnoses, transplant status, initial severity of illness (as measured by Pediatric Risk of Mortality [PRISM] III score [20]), infection status, and mortality.
Data analysis
Descriptive statistics are shown as median (interquartile range [IQR]) or mean (± standard error of mean [SEM]) where appropriate according to the D’Agostino and Pearson omnibus normality test. Simple comparisons were made using the Mann–Whitney rank-sum test. Relative risks with 95% confidence intervals (CI) were calculated for development of nosocomial infection and death (Prism4; GraphPad Software Inc., San Diego, CA). Differences between groups over time were analyzed on log-transformed data using two-factor analysis of variance (ANOVA) or repeated-measures (RM) ANOVA. Fisher’s exact test was used to evaluate categorical variables between groups (as above). p-Value < 0.05 was considered to represent statistical significance.
Results
Study 1
Seventy patients were enrolled in the observational study with median age of 5 (1–15) years. Diagnoses included primary sepsis (n = 30), posttransplant organ dysfunction (n = 8), pneumonia (n = 16), liver failure (n = 6), and heart failure (n = 5). The number of organ failures ranged from 2 to 5 at time of enrollment (OFI = 2, n = 30; OFI = 3, n = 27; OFI = 4, n = 10; OFI = 5, n = 3). Mean PRISM III score at onset of MODS was 13 ± 0.7. Overall mortality was 23% (16/70). Eight control subjects were also enrolled (median age 9.8 [8–12] years). Median ex vivo LPS-induced TNFα response was 1,386 (900–2,172) pg/mL, and plasma IL-6 levels were <10 pg/mL in the control subjects.
Ex vivo LPS-induced TNFα response <200 pg/mL on day 7 of MODS was associated with development of nosocomial infection (p = 0.01; Fig. 1, top) and death (p = 0.0007; Fig. 1, bottom). These relationships were similar to those seen with HLA-DR expression, using the previously reported cutoff of 30% (p = 0.0002 for nosocomial infection, p = 0.01 for death). We did not find these relationships with either biomarker at the earlier time points (1 or 3 days). Given the highly standardized nature of the ex vivo LPS-induced TNFα response procedure in our laboratories, we elected to use this method for the remainder of this multicenter study.
Monocyte HLA-DR expression and ex vivo LPS-induced TNFα response in 27 children with MODS. These children underwent measurement of both biomarkers on day 7 of MODS. Top Children who went on to develop nosocomial infection (gray squares) had lower ex vivo LPS-induced TNFα production and monocyte HLA-DR expression compared with children who recovered without developing infection (open circles). Bottom Children who went on to die (gray squares) similarly demonstrated lower immune function compared with survivors (open circles). The dashed lines show the biomarker threshold levels which define immunoparalysis. Patients below these thresholds had greater risk of developing nosocomial infection and death (p < 0.01)
Twenty-four out of the 70 multiple organ dysfunction patients (34%) developed immunoparalysis. These patients had increased relative risks for development of nosocomial infection and death (Tables 1, 2). Overall, 27 patients (39%) developed nosocomial infection (Table 2). Patients who developed persistent nosocomial infection all had immunoparalysis for 1 week or longer, with increased systemic IL-6 levels compared with patients in whom nosocomial infection resolved more rapidly. Patients in whom nosocomial infection resolved before 7 days did not have immunoparalysis and had both higher LPS-stimulated TNFα response and lower systemic IL-6 levels (p < 0.0001 and 0.01, respectively, by two-factor analysis of variance). Patients with immunoparalysis throughout the first 7 days after their first positive culture had 5.8-fold higher relative risk (95% CI 1.6–21, p = 0.002) of having persistent nosocomial infection compared with patients whose TNFα response improved over time.
Nine transplant recipients developed immunoparalysis and nosocomial infection. In children who underwent rapid tapering (n = 3), the TNFα response tended to increase within 7 days compared with children who did not undergo tapering (n = 6) (ex vivo LPS-induced TNFα response: 199 [184–938] versus 38 [24–111] pg/mL; p = 0.077, two-way ANOVA). This was not associated with an increase in plasma IL-6 levels (5 [5–53] versus 87 [10–776] pg/mL; p = 0.16, two-way ANOVA). All patients who underwent tapering had infection resolution within 1 week and survived. Among the six patients who did not undergo tapering, only one had infection resolution and survived (3/3 versus 1/6, p < 0.05).
Study 2
Seventeen patients were enrolled. Three patients had ex vivo LPS-induced TNFα response >160 pg/mL. According to study design, these patients were not randomized. All three survived without any episodes of nosocomial infection. The remaining 14 were randomized to the GM-CSF or standard therapy arms (Fig. 2). None of these patients developed white blood cell count >30,000/mm3. Children randomized to the standard therapy arm required more than 7 days in the PICU to reverse immunoparalysis. Similar to in study 1, all seven of these standard care patients developed nosocomial infection, with one child developing two separate nosocomial infections at two separate times. Two of these seven patients died. In contrast, the seven children randomized to GM-CSF therapy showed reversed immunoparalysis in <7 days and experienced no nosocomial infections (p < 0.05) or deaths. Treatment with GM-CSF did not increase systemic IL-6 levels. No GM-CSF-related adverse events were observed.
GM-CSF therapy in nontransplant, nonneutropenic patients resulted in increased LPS sensitivity and prevention of development of nosocomial infection without increasing systemic inflammation. Children with ex vivo LPS-induced TNFα production <160 pg/mL on day 3 of MODS were randomized to receive GM-CSF or standard therapy. Top Children receiving GM-CSF (open circles, n = 7) demonstrated more rapid recovery of ex vivo LPS-induced TNFα production >200 pg/mL compared with children receiving standard therapy alone (filled squares, n = 7) (p = 0.001; two-way RM-ANOVA). Bottom The seven patients who received standard care all developed nosocomial infections, with one patient developing two, for a total of eight nosocomial infections (three Gram negative, five fungal). At day 7 and 14, six patients had active infections, and at day 2, three patients had active infections. No nosocomial infections were observed in the GM-CSF-treated group (p < 0.05)
Discussion
The results of our study show that, similar to the adult experience, children with MODS commonly develop immunoparalysis with increased susceptibility to nosocomial infection and death. Persistence of nosocomial infection was observed in children with persistent immunoparalysis. Although nosocomial infections were also observed in children without immunoparalysis, they occurred at a lesser rate and resolved more rapidly. Our observation that GM-CSF therapy facilitated reversal of immunoparalysis and prevented secondary infection in children with severe MODS supports the possibility that reversal of immunoparalysis in critically ill children can be both clinically attainable and important. This finding is consistent with the recent randomized adult sepsis trial of GM-CSF in which this therapy both reversed immunoparalysis and reduced morbidity end points [14].
Over the last 10 years, the literature has suggested that restoration of capacity to mount an innate immune response is associated with recovery from critical illness in adults. While transient downregulation of the proinflammatory response is common after an insult such as sepsis or trauma in adults, this compensatory antiinflammatory response syndrome (CARS) promptly gives way to immunologic homeostasis [21] in patients who recover. In a subset of patients, however, a state of antiinflammatory dominance persists, known as “immunoparalysis” [5]. This phenotype has been defined by prolonged depression of the biomarkers monocyte HLA-DR expression and ex vivo LPS responsiveness, with subsequent increased risk of development of nosocomial infection and death in the settings of sepsis, trauma, major surgery, and pancreatitis [7, 9, 11, 12, 22–25]. Similarly, we found that children with immunoparalysis and MODS had increased risks of nosocomial infection and mortality.
An important question which remains to be answered is whether maneuvers that restore biomarker levels above these critical thresholds can reverse immunoparalysis and reduce the risks of developing secondary infection and death. For transplant patients receiving exogenous immunosuppression, rapid tapering of immunosuppression in the setting of sepsis-induced immunoparalysis has been associated with patient survival with improved HLA-DR expression and preservation of graft function [8]. In our observational cohort of transplant patients, rapid tapering was associated with fewer secondary infections and deaths. In the nontransplant setting, monocyte suppression has been shown to be reversible in vitro using agents such as IFNγ, GM-CSF, and neutralizing antibodies against the antiinflammatory cytokine IL-10 [26–30]. In particular, GM-CSF is capable of upregulating monocyte responsiveness of existing monocytes rather than only encouraging increased bone marrow production of new monocytes. Recombinant immune-stimulating cytokines including IFNγ and GM-CSF have been administered to critically ill adults, with promising results [10, 14, 17, 18].
We elected to use GM-CSF for the randomized, controlled trial portion of our study, because GM-CSF is Food and Drug Administration (FDA) approved and commonly used in children. Similar to other immunomodulation studies of GM-CSF [14, 17, 18, 31], we used a dosage (125 μg/m2/day) that is substantially lower than the 250 μg/m2/day dosage used by oncologists for bone marrow reconstitution. As an added safety measure, we administered the drug as prolonged IV infusion (over at least 12 h) to avoid the capillary leak and edema that have been reported with rapid IV infusion [32]. We also enriched our study population by using inclusion criteria associated with 100% nosocomial infection risk in study 1, namely ≥3 organs failing and ex vivo TNFα response <160 pg/ml at 3 days. In this selected population, GM-CSF therapy more rapidly increased the TNFα response above the critical threshold of 200 pg/mL and prevented development of nosocomial infection. Restoration of TNFα response was not associated with an increase in plasma levels of IL-6, a marker of increased inflammation and death in sepsis [19, 33]. Because IL-6 can be produced by nonimmune tissues including vascular endothelium, it is possible that restoration of monocyte function which parallels reduction in infection can reduce overall systemic inflammation [34, 35]. More work is required to explore this possibility.
There are limitations to consider when evaluating our study. Because MODS patients represent a heterogeneous patient population, the underlying mechanisms contributing to immunoparalysis are likely to be varied within this group and in centers with different categories of patients. Despite the fact that this study is the largest of its kind, continued efforts to increase the sample size in this field of study are needed to further test our observations. We also performed this study using the ex vivo TNFα biomarker not the HLA-DR biomarker. In our view, the ex vivo LPS-induced TNFα response assay has certain advantages over quantification of monocyte HLA-DR expression using flow cytometry. The LPS stimulation procedure can be done using highly standardized procedures, and TNFα can be quantified on a highly automated platform with very low interassay variance, as was done in this study. Flow cytometry results can depend on cytometer settings, lot-to-lot variability in fluorochromes, and, of course, cytometer availability. We also did not present the TNFα response per monocyte. Some patients with low ex vivo TNFα response had low monocyte count, while others did not. The per-monocyte measure conferred no advantage in identifying nosocomial infection risk over the whole-blood measure. We recognize that either technique may not be suitable in all centers and will entail a certain cost. The generalized feasibility and utility of immune monitoring remain to be evaluated.
Conclusions
Our study supports immunoparalysis as a potentially reversible risk factor for development of nosocomial infection in children with MODS. Further studies are warranted to evaluate the benefit of using ex vivo whole-blood TNFα response as a biomarker to guide immune phenotype-directed therapies for prevention and reversal of nosocomial infections in pediatric MODS patients.
References
Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, Gauvin F, Hubert P, Lacroix J (2005) Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Resp Crit Care Med 171:348–353
Pizzo PA, Rubin M, Freifeld A, Walsh TJ (1991) The child with cancer and infection. I. Empiric therapy for fever and neutropenia, and preventive strategies. J Pediatr 119:679–694
Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA (2005) Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 174:3765–3772
Hoffman JA, Weinberg KI, Azen CG, Horn MV, Dukes L, Starnes VA, Woo MS (2004) Human leukocyte antigen-DR expression on peripheral blood monocytes and the risk of pneumonia in pediatric lung transplant recipients. Transpl Infect Dis 6:147–155
Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, Docke WD, Kox WJ (1996) Monocyte deactivation–rationale for a new therapeutic strategy in sepsis. Intensive Care Med 22(Suppl 4):S474–S481
Monneret G, Finck ME, Venet F, Debard AL, Bohe J, Bienvenu J, Lepape A (2004) The anti-inflammatory response dominates after septic shock: Association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95:193–198
Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC Jr (1990) Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg 77:204–207
Reinke P, Volk HD (1992) Diagnostic and predictive value of an immune monitoring program for complications after kidney transplantation. Urol Int 49:69–75
Ho YP, Sheen IS, Chiu CT, Wu CS, Lin CY (2006) A strong association between down-regulation of HLA-DR expression and the late mortality in patients with severe acute pancreatitis. Am J Gastroenterol 101:1117–1124
Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W (1997) Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat Med 3:678–681
Ploder M, Pelinka L, Schmuckenschlager C, Wessner B, Ankersmit HJ, Fuerst W, Redl H, Roth E, Spittler A (2006) Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock 25:129–134
Allen ML, Hoschtitzky JA, Peters MJ, Elliott M, Goldman A, James I, Klein NJ (2006) Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med 34:2658–2665
Woiciechowsky C, Asadullah K, Nestler D, Schoning B, Glockner F, Docke WD, Volk HD (1998) Diminished monocytic HLA-DR expression and ex vivo cytokine secretion capacity in patients with glioblastoma: Effect of tumor extirpation. J Neuroimmunol 84:164–171
Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD (2009) GM-CSF to reverse sepsis-associated immunosuppression: A double-blind randomized placebo-controlled multicenter trial. Am J Respir Crit Care Med 180:640–648
Kox WJ, Bone RC, Krausch D, Docke WD, Kox SN, Wauer H, Egerer K, Querner S, Asadullah K, von Baehr R, Volk HD (1997) Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: Proof of principle. Arch Intern Med 157(4):389–393
Nakos G, Malamou-Mitsi VD, Lachana A, Karassavoglou A, Kitsiouli E, Agnandi N, Lekka ME (2002) Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med 30:1488–1494
Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, Schneider CG, Pothmann W, Brassel AK, Schulte Am Esch J (2003) Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med 29:646–651
Rosenbloom AJ, Linden PK, Dorrance A, Penkosky N, Cohen-Melamed MH, Pinsky MR (2005) Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest 127:2139–2150
Doughty LA, Kaplan SS, Carcillo JA (1996) Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med 24:1137–1143
Pollack MM, Patel KM, Ruttimann UE (1996) Prism iii: An updated pediatric risk of mortality score. Crit Care Med 24:743–752
Bone RC (1996) Sir Isaac Newton, sepsis, sirs, and cars. Crit Care Med 24:1125–1128
Volk HD, Reinke P, Docke WD (2000) Clinical aspects: From systemic inflammation to ‘immunoparalysis’. Chem Immunol 74:162–177
Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P (2006) Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32:1175–1183
Volk HD, Reinke P, Docke WD (1999) Immunological monitoring of the inflammatory process: Which variables? When to assess? Eur J Surg Suppl 584:70–72
Flach R, Majetschak M, Heukamp T, Jennissen V, Flohe S, Borgermann J, Obertacke U, Schade FU (1999) Relation of ex vivo stimulated blood cytokine synthesis to post-traumatic sepsis. Cytokine 11:173–178
Spagnoli GC, Juretic A, Rosso R, Van Bree J, Harder F, Heberer M (1995) Expression of HLA-DR in granulocytes of polytraumatized patients treated with recombinant human granulocyte macrophage-colony-stimulating factor. Hum Immunol 43:45–50
Lendemans S, Kreuzfelder E, Waydhas C, Schade FU, Flohe S (2007) Differential immunostimulating effect of granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) and interferon gamma (IFN gamma) after severe trauma. Inflamm Res 56:38–44
Bundschuh DS, Barsig J, Hartung T, Randow F, Docke WD, Volk HD, Wendel A (1997) Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol 158:2862–2871
Flohe S, Lendemans S, Selbach C, Waydhas C, Ackermann M, Schade FU, Kreuzfelder E (2003) Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit Care Med 31:2462–2469
Fumeaux T, Pugin J (2002) Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Resp Crit Care Med 166:1475–1482
Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW (2002) A randomized phase ii trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Resp Crit Care Med 166:138–143
Herrmann F, Schulz G, Lindemann A, Meyenburg W, Oster W, Krumwieh D, Mertelsmann R (1988) Yeast-expressed granulocyte-macrophage colony-stimulating factor in cancer patients: A phase 1b clinical study. Behring Inst Mitt 83:107–118
Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC (2007) Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (genims) study. Arch Intern Med 167:1655–1663
Bultinck J, Brouckaert P, Cauwels A (2006) The in vivo contribution of hematopoietic cells to systemic TNF and IL-6 production during endotoxemia. Cytokine 36:160–166
Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D (1995) Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem 270:11463–11471
Acknowledgments
The authors would like to acknowledge Patricia Guittar, RN for her assistance with subject identification and enrollment. This study was funded in part by NCRR 3M01RR0056GCRC (J.A.C.), NICHD K12 HD43372 (M.W.H.), NHLBI K08 HL085525 (M.W.H.), Deutsche Froschungsgemeinschaft SFB 421 (H.D.V.), and The Research Institute at Nationwide Children’s Hospital (M.W.H.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hall, M.W., Knatz, N.L., Vetterly, C. et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 37, 525–532 (2011). https://doi.org/10.1007/s00134-010-2088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2088-x