Abstract
Purpose
To describe the outcomes of patients with bronchiectasis and acute respiratory failure (ARF) treated with noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) after a failure of conservative measures, and to identify the predictors of hospital mortality and NIV failure.
Methods
Retrospective review of bronchiectatic patients on NIV (n = 31) or IMV (n = 26) for ARF over 8 years in a medical intensive care unit (ICU) experienced in NIV.
Results
At baseline, the NIV group had more patients with acute exacerbations without identified precipitating factors (87.1 vs. 34.6%, p < 0.001), higher pH (mean 7.25 vs. 7.18, p = 0.008) and PaO2/FiO2 ratio (mean 249.4 vs. 173.2, p = 0.02), and a trend towards a lower APACHE II score (mean 25.3 vs. 28.4, p = 0.07) than the IMV group. There was no difference in hospital mortality between the two groups (25.8 vs. 26.9%, p > 0.05). The NIV failure rate (need for intubation or death in the ICU) was 32.3%. Using logistic regression, the APACHE II score was the only predictor of hospital mortality (OR 1.19 per point), and the PaO2/FiO2 ratio was the only predictor of NIV failure (OR 1.02 per mmHg decrease).
Conclusions
The hospital mortality of patients with bronchiectasis and ARF approximates 25% and is predicted by the APACHE II score. When selectively applied, NIV fails in one-third of the patients, and this is predicted by hypoxemia. Our findings call for randomised controlled trials to compare NIV versus IMV in such patients.
Similar content being viewed by others
Introduction
Although once thought of as an orphan disease [1], it is now evident that the prevalence of non-cystic fibrosis (CF) bronchiectasis remains high [2, 3]. Patients with non-CF bronchiectasis often die of causes related to bronchiectasis and acute respiratory failure (ARF) [4–7]. However, to the best of our knowledge, there are only two small retrospective reports to date that describe the course of respiratory failure in the intensive care unit (ICU) for such patients [8, 9]. Meanwhile, although noninvasive ventilation (NIV) is increasingly being used for various conditions such as in chronic obstructive pulmonary disease (COPD) [10], no data exist that describe the feasibility of noninvasive as opposed to invasive mechanical ventilation (IMV) for ARF associated with bronchiectasis.
Accordingly, the aims of our study were firstly to describe the outcomes of ARF in bronchiectasis treated with NIV and IMV, and secondly to identify the predictors of hospital mortality and the failure of NIV in these patients.
Methods and materials
Patient population
This study was approved by our university hospital’s institutional review board with a waiver of informed consent. Using admission and discharge records, we included all consecutive adult patients above 21 years of age with bronchiectasis who were on NIV or IMV for ARF in our medical ICU between January 2000 and November 2007. Only the first episode of ventilatory support and ICU stay for each patient was included. The inclusion and exclusion criteria applied to both patients on NIV and IMV.
We defined bronchiectasis based on: (1) symptoms of chronic sputum production or cough for ≥1 year before the ICU admission and (2) the following computed tomography (CT) findings: nontapering bronchus with internal diameter ≥110% compared to the adjacent pulmonary artery or visible bronchi within 1 cm of the costal pleural surface or adjacent to the mediastinal pleural surface [3, 11, 12]. We gave preference to high-resolution (HRCT) rather than conventional CT scans. In the absence of CT scans, bronchiectasis was only defined when (1) clinical features were ascertained to be consistent with bronchiectasis by the attending respiratory physician and (2) chest radiograph findings were ascertained to be consistent with bronchiectasis by a radiologist.
We defined ARF as either (1) hypoxemic [partial pressure of arterial oxygen (PaO2) <60 mmHg on room air] or (2) hypercapnic [partial pressure of arterial carbon dioxide (PaCO2) >50 mmHg] respiratory failure with or without acidemia (pH < 7.35), plus (3) at least two of the following: worsening dyspnea, respiratory rate >25 breaths/min, use of the accessory muscles of respiration, cyanosis and reduced alertness (any state ranging from drowsiness simulating light sleep to stupor and coma) [13].
We excluded patients who were ventilated for reasons other than ARF such as non-pulmonary sepsis or airway protection for massive haemoptysis. To ensure no overlap between bronchiectasis and COPD, we excluded all patients in a COPD registry that our ICU has kept since 1999 [14, 15]. The diagnosis of COPD was based on the Global Initiative for Chronic Obstructive Lung Disease guidelines [16]. We do not perform routine HRCT scans for COPD patients and therefore did not include any COPD patients with non-clinically significant bronchiectasis detected on HRCT scans in this study.
Data collection
We recorded the following via medical record review onto standardised data collection forms: demographics, the underlying cause and predominant radiological type of bronchiectasis (cylindrical, cystic or varicose [17]) and the use of long-term oxygen therapy (LTOT).
We grouped the causes of ARF into: (1) acute exacerbation of bronchiectasis without identified precipitating factors and (2) specific causes superimposed on bronchiectasis. These causes included pneumonia (new chest radiograph infiltrate plus fever, leucocytosis or leucopenia [18]), heart failure (pulmonary or peripheral oedema and an abnormal echocardiogram [19]), haemoptysis, tuberculosis (Mycobacterium tuberculosis in the sputum [20]) and lung abscess (cavity on the chest radiograph with an air-fluid level [21]). We recorded any symptoms of increased sputum production and signs of reduced alertness, vital signs and arterial blood gas measurements before and 1 h after ventilation, ventilator settings during the first hour, culture results, the Acute Physiology and Chronic Health Evaluation (APACHE) II score, if the patients were admitted to the ICU from the emergency department or the general ward, and any do-not-intubate orders.
Treatment regimes
Our ICU and emergency department have a common protocol for the initial support of ARF patients. Specific therapies include nebulised salbutamol and ipratropium bromide and systemic steroids for wheezing, empiric broad-spectrum antibiotics and chest physiotherapy for respiratory secretions. Patients who failed such conservative measures and had persistent ARF as defined above were put on NIV or IMV.
We recommend the following indications for endotracheal intubation and IMV: respiratory arrest or pauses, agitation requiring sedation, systolic blood pressure <70 mmHg and new-onset multiorgan failure involving ≥2 non-pulmonary organs—ultimately, clinical judgment is applied. Reduced alertness from hypercapnia alone was not a definite indication for intubation [22]. We start with assist control volume-limited ventilation, tidal volumes of 6 ml/kg predicted body weight, and respiratory rate and positive end-expiratory pressure (PEEP) set to avoid dynamic hyperinflation. We switch to a pressure support of 15 cmH2O when the patients improve, while keeping the respiratory rate <30/min. We utilise heat and moisture exchangers, inline suctioning for secretions, metered dose inhalers for bronchodilator delivery through the ventilator circuit, and a nursing and respiratory therapist-directed sedation and weaning protocol with daily spontaneous awakening and breathing trials [23].
We consider NIV for patients with persistent ARF without the indications for IMV. We use the BiPAP Vision in the spontaneous/timed mode and a secured Total Face Mask or Spectrum Disposable Full Face Mask (Respironics, Murrysville, PA) [14]. We start with an inspiratory positive airway pressure (IPAP) of 18 cmH2O and an expiratory positive airway pressure (EPAP) of 4 cmH2O, making adjustments to keep the respiratory rate <25/min. We apply NIV for as long as tolerated during the first 24 h without routine humidification and thereafter based on the patients’ needs, interrupting NIV for secretion clearance and bronchodilator nebulisation [24]. In addition to the above indications for intubation, we consider intubation for the following after 1 h of NIV: respiratory rate >35/min, PaO2 <60 mmHg with the fraction of inspired oxygen (FiO2) ≥ 0.5, pH < 7.26 [25], drowsiness or inability to clear secretions.
Outcomes
These included ICU and hospital mortality and length of stay, and 28-day ventilator-free days (the number of days between the start of ventilation and day 28 without NIV and IMV; patients who died before day 28 had zero ventilator-free days [26]). For the NIV group, we defined NIV failure as the need for intubation or death within the same ICU admission. For the IMV group, we defined weaning failure if the patient recurrently failed the spontaneous breathing trial and was not extubated, was tracheotomised, or required reintubation or died within 48 h of extubation [27]. We also documented if the patient was placed on NIV within 48 h of extubation.
Statistical analysis
We expressed variables as frequencies (percentages), means ± standard deviations (SD) and medians (interquartile ranges). We performed two-sided univariate analyses using the chi-square test, Fisher’s exact test, t test, paired t test and Mann–Whitney U test where appropriate. Comparisons were made between the NIV and IMV groups for the following variables: age, sex, cause of bronchiectasis and ARF, LTOT, sputum production, reduced alertness, vital signs, blood gas measurements, APACHE II score, the original patient location and do-not-intubate orders. To identify the predictors of hospital mortality, we first performed a univariate analysis of the type of ventilation and these variables, where available, to compare survivors versus nonsurvivors. We entered variables with p values <0.15 into a forward stepwise logistic regression analysis using an entry level of 0.05 and a removal level of 0.10. We performed a similar analysis to identify the predictors of NIV failure. A p value of <0.05 was considered significant. We used SPSS version 15.0 (SPSS Inc., Chicago, IL).
Results
Patient characteristics
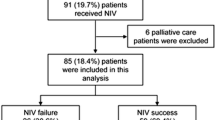
There were 31 patients in the NIV group and 26 in the IMV group (Fig. 1). Table 1 shows their baseline characteristics. No differences in demographic data and underlying causes of bronchiectasis existed between these groups. Forty-one patients’ CT scans were available for review, of which 26 were HRCT scans. The predominant type of bronchiectasis was cylindrical, cystic and varicose in 24, 14 and 3 patients, respectively.
Most patients treated with NIV had acute exacerbations of bronchiectasis without identified precipitating factors, while most patients treated with IMV had specific triggers including pneumonia (Table 1). The majority (48 patients) in both groups had hypercapnic respiratory failure with acidemia. At baseline, while there were no differences in vital signs and frequency of reduced alertness, the NIV group had a greater frequency of increased sputum production, and was less acidemic and hypoxemic than the IMV group. The APACHE II score was numerically but not significantly lower in the NIV group.
Ventilator settings and effects
During the first hour of ventilation, the mean set IPAP, EPAP and FiO2 were respectively, 17.7 ± 3.4, 6.8 ± 2.1 cm H2O and 0.40 ± 0.15 for the NIV group. The mean tidal volumes, set PEEP and FiO2 were respectively 419 ± 85 ml, 7.9 ± 2.7 cmH2O and 0.64 ± 0.21 for the IMV group. Figure 2 describes the effect of NIV and IMV on the vital signs and blood gas measurements. Both reduced the respiratory rate and PaCO2 significantly, although only NIV led to a significant increase in pH. There was however no difference in the magnitude of change caused by NIV versus IMV in any variable.
Vital signs and arterial blood gas measurements before and 1 h into ventilatory support. Dotted lines represent the noninvasive ventilation (NIV) group, while bold lines represent the invasive mechanical ventilation (IMV) group. Mean values are shown. Error bars represent the 95% confidence intervals. *Significant change in values before and during (at 1 h) ventilatory support (p < 0.05, Student’s t test). NS not significant. There was no significant difference in the magnitude of change caused by NIV and IMV in all parameters
Outcomes
The patient outcomes are shown in Table 2. The NIV group had a shorter ICU length of stay than the IMV group (p = 0.002). Although the NIV group had more 28-day ventilator-free days and a shorter hospital length of stay, these were not statistically significant.
Fifteen patients died in the hospital from the following causes: cardiac arrest associated with respiratory failure (3 NIV, 1 IMV), multiorgan (including respiratory) failure from pneumonia (4 NIV, 6 IMV) and acute gastrointestinal bleed (1 NIV). There were no differences in ICU or hospital mortalities between the two groups (Table 2). Among all the variables studied on univariate analysis, the APACHE II score was the only one that differed between survivors and nonsurvivors (Table 3). In a logistic regression analysis that included the APACHE II score, the cause of ARF, heart rate and PaO2/FiO2 ratio, the APACHE II score was the only independent predictor of hospital mortality.
Figure 1 details the immediate outcomes of weaning and NIV in the two groups. In the NIV group, the actual duration of NIV excluding breaks was 13 h (4.8–20.0) and 16.5 h (4.8–31.5) during the first 24 h and throughout the ICU stay, respectively. There were ten NIV failures, including two deaths with do-not-intubate orders and eight intubations. Reasons for intubation included deterioration in blood gas measurements in six patients, worsening respiratory rate, heart rate and blood pressure in three patients and increasing drowsiness in three patients (some patients had more than one reason). No patient was intubated for the inability to clear secretions. On univariate analysis, patients with identified precipitating factors, worse hypercapnia and hypoxemia were more likely to have NIV failure (Table 4). Logistic regression analysis showed that the PaO2/FiO2 ratio was the only independent predictor of NIV failure.
Discussion
Our study’s key findings are as follows. One-quarter of our patients with bronchiectasis and ARF died in the hospital. This mortality was independently predicted by the APACHE II score. Physicians often reserved NIV for patients with acute exacerbations without identified precipitating factors and who were less acidemic and hypoxemic. There was no difference in the magnitude of change in physiological variables caused by NIV versus IMV over 1 h. Although the NIV group had a shorter ICU length of stay, one-third of these patients failed NIV, and this was independently predicted by hypoxemia.
Few data exist on the outcomes of non-CF bronchiectasis. Long-term mortality varies between studies from 42% over 4 years to 19% over 14 years [4–7], and respiratory failure is a common cause of death. Nevertheless, only two other retrospective studies have focussed on patients with bronchiectasis and ARF in the ICU. These studies by Dupont et al. (48 patients) and Alzeer et al. (35 patients) reported an ICU mortality of 19 and 34%, respectively [8, 9]. The hospital mortality of 26.3% in our cohort was consistent with these studies’ findings, and the APACHE II score was the only predictor of mortality on both univariate and multivariate analyses. This finding is similar to those of Alzeer et al. [9] who found a high APACHE II score to be a prognostic factor on univariate analysis.
No previous study has evaluated the feasibility of NIV in ARF associated with non-CF bronchiectasis in comparison with IMV. This is despite the fact that NIV is increasingly being used for other causes of ARF [10], that domiciliary nasal NIV has been described for chronic respiratory failure in bronchiectasis [28–30] and that data exist on NIV in CF [31]. The studies by Dupont et al. and Alzeer et al. reported using NIV in 13 and 20 patients, respectively, but did not specify the characteristics and outcomes of these patients. In our study, NIV was used for more than half of the patients. This high frequency of use is likely related to our ICU’s familiarity with NIV and the presence of acute hypercapnic respiratory failure in most patients. The mean pH and PaCO2 were 7.25 and 77.8 mmHg, respectively, in the NIV group, and previous data suggest that NIV may be more effective in hypercapnic than hypoxemic respiratory failure [10]. Our physicians did however select patients with moderate rather than severe acidemia and hypoxemia for NIV, as severe physiologic derangements are associated with NIV failure [32, 33]. Our physicians also used NIV more frequently for exacerbations without identified precipitants than for specific triggers like pneumonia, and the varied causes of ARF complicate our findings. Although one study suggested a high success rate for NIV in COPD patients with pneumonia [34], most other data on NIV for pneumonia have been disappointing [14, 32, 33, 35]. In addition, NIV was used for 11 patients with do-not-intubate orders, this being an increasingly prevalent practice [10]. Only two of these patients died, which is a better outcome than that reported in other studies on do-not-intubate patients [36, 37]. Bearing these caveats about patient selection in mind, one-third of our patients had NIV failure, which was higher than the 20% failure rate reported in COPD in a systematic review [38]. Failure of NIV was indeed more common in patients with precipitating factors like pneumonia and hypercapnia on univariate analysis, and was independently predicted by the severity of hypoxemia on multivariate analysis.
Our findings nevertheless suggest that NIV is a viable alternative to IMV in selected patients with ARF associated with bronchiectasis. An excess of respiratory secretions is often quoted as a contraindication for NIV [10], and this should theoretically suggest that bronchiectatic patients are poor NIV candidates. This was however not the case in our study, where the NIV group had more increased sputum production than the IMV group, and where no patients on NIV were intubated due to the inability to clear secretions. The benefits of NIV in bronchiectasis remain attractive. Like COPD, bronchiectasis is often associated with slow-to-resolve respiratory failure, thus the need to avoid prolonged IMV and the resultant ventilator-associated pneumonia. Our data suggest that NIV may indeed be used for this purpose in patients who fail initial conservative measures, a situation that is also witnessed in COPD patients [10, 39]. In our cohort, NIV resulted in the same magnitude of change in vital signs and blood gas measurements as IMV. Although a cause-effect relationship cannot be established, NIV was associated with a shorter ICU length of stay than IMV, a benefit also seen in COPD [38, 40].
We acknowledge the limitations of our study. First, due to its retrospective nature, important details including premorbid and nutritional status, comorbidities, a detailed smoking history, lung function tests, the exact level of reduced alertness using scales like the Glasgow Coma Scale, complications like skin breakdown in the NIV group, ventilator-associated pneumonia and atelectasis from mucus plugging, and 28-day and long-term mortality were lacking. Second, computed tomography scans were unavailable for some patients; we could only include these patients if the clinical diagnosis of bronchiectasis was agreed upon by both the attending respiratory physician and the radiologist interpreting the chest radiograph. Third, although this is the largest ever ICU study on ARF in bronchiectasis, the sample size remains small and likely contributed to the lack of statistical significance when changes in certain parameters with ventilatory support were assessed (Fig. 2). Fourth, our multivariate analyses must be interpreted with caution. Although parameters after 1 h of NIV often predict NIV failure [10], we did not include them in the multivariate (or univariate) analyses because the small sample size prohibited a valid model with many variables and because we were more interested in predictors of NIV failure at baseline. In addition, there were more patients with pneumonia and a trend toward higher APACHE II scores in the IMV group, but more patients with do-not-intubate orders in the NIV group. While all these variables may predict mortality, the APACHE II score was the only independent predictor in our model. The various other confounders may have been inadequately accounted for given the small sample size. As such, the fact that the type of ventilatory support did not feature as a predictor of mortality should merely be seen as hypothesis-generating.
In conclusion, the hospital mortality of patients with bronchiectasis and ARF approximates 25% and is independently predicted by the APACHE II score. When selectively applied, NIV fails in one-third of the patients, and this is independently predicted by hypoxemia. Our findings call for randomised controlled trials to compare NIV versus IMV in such patients.
References
Barker AF, Bardana EJ Jr (1988) Bronchiectasis: update of an orphan disease. Am Rev Respir Dis 137:969–978
Tsang KW, Tipoe GL (2004) Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis 8:691–702
O’Donnell AE (2008) Bronchiectasis. Chest 134:815–823
Loebinger MR, Wells AU, Hansell DM, Chinyanganya N, Devaraj A, Meister M, Wilson R (2009) Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 34:843–849
Onen ZP, Gulbay BE, Sen E, Yildiz OA, Saryal S, Acican T, Karabiyikoglu G (2007) Analysis of the factors related to mortality in patients with bronchiectasis. Respir Med 101:1390–1397
Keistinen T, Saynajakangas O, Tuuponen T, Kivela SL (1997) Bronchiectasis: an orphan disease with a poorly understood prognosis. Eur Respir J 10:2784–2787
Ellis DA, Thornley PE, Wightman AJ, Walker M, Chalmers J, Crofton JW (1981) Present outlook in bronchiectasis: clinical and social study and review of factors influencing prognosis. Thorax 36:659–664
Dupont M, Gacouin A, Lena H, Lavoue S, Brinchault G, Delaval P, Thomas R (2004) Survival of patients with bronchiectasis after the first ICU stay for respiratory failure. Chest 125:1815–1820
Alzeer AH, Masood M, Basha SJ, Shaik SA (2007) Survival of bronchiectatic patients with respiratory failure in ICU. BMC Pulm Med 7:17
Garpestad E, Brennan J, Hill NS (2007) Noninvasive ventilation for critical care. Chest 132:711–720
Naidich DP, McCauley DI, Khouri NF, Stitik FP, Siegelman SS (1982) Computed tomography of bronchiectasis. J Comput Assist Tomogr 6:437–444
McGuinness G, Naidich DP (2002) CT of airways disease and bronchiectasis. Radiol Clin North Am 40:1–19
Ropper AH (2008) Coma. In: Fauci AS (ed) Harrison’s principles of internal medicine, 17th edn. McGraw-Hill, New York, pp 1714–1719
Phua J, Kong K, Lee KH, Shen L, Lim TK (2005) Noninvasive ventilation in hypercapnic acute respiratory failure due to chronic obstructive pulmonary disease versus other conditions: effectiveness and predictors of failure. Intensive Care Med 31:533–539
Ai-Ping C, Lee KH, Lim TK (2005) In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective study. Chest 128:518–524
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176:532–555
Reid LM (1950) Reduction in bronchial subdivision in bronchiectasis. Thorax 5:233–247
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29:2388–2442
American Thoracic Society; Centers for Disease Control and Prevention; Infectious Disease Society of America (2000) Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161:1376–1395
Muller NL, Fraser RS, Colman NC, Pare PD (2001) Radiologic diagnosis of diseases of the chest. W. B. Saunders, Philadelphia
Scala R, Naldi M, Archinucci I, Coniglio G, Nava S (2005) Noninvasive positive pressure ventilation in patients with acute exacerbations of COPD and varying levels of consciousness. Chest 128:1657–1666
Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet 371:126–134
Mukhopadhyay A, Dela Pena E, Wadden B, Procyshyn M, Lim TK (2009) Effects of inhalational bronchdilator treatment during non-invasive ventilation in severe COPD exacerbations. J Crit Care 24:474, e1–5
Confalonieri M, Garuti G, Cattaruzza MS, Osborn JF, Antonelli M, Conti G, Kodric M, Resta O, Marchese S, Gregoretti C, Rossi A (2005) A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 25:348–355
Schoenfeld DA, Bernard GR (2002) Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 30:1772–1777
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Simonds AK, Elliott MW (1995) Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax 50:604–609
Gacouin A, Desrues B, Lena H, Quinquenel ML, Dassonville J, Delaval P (1996) Long-term nasal intermittent positive pressure ventilation (NIPPV) in sixteen consecutive patients with bronchiectasis: a retrospective study. Eur Respir J 9:1246–1250
Benhamou D, Muir JF, Raspaud C, Cuvelier A, Girault C, Portier F, Menard JF (1997) Long-term efficiency of home nasal mask ventilation in patients with diffuse bronchiectasis and severe chronic respiratory failure: a case-control study. Chest 112:1259–1266
Moran F, Bradley JM, Piper AJ (2009) Non-invasive ventilation for cystic fibrosis. Cochrane Database Syst Rev CD002769
Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, Pelaia P, Principi T, Gregoretti C, Beltrame F, Pennisi MA, Arcangeli A, Proietti R, Passariello M, Meduri GU (2001) Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 27:1718–1728
Ambrosino N, Foglio K, Rubini F, Clini E, Nava S, Vitacca M (1995) Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: correlates for success. Thorax 50:755–757
Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G (1999) Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med 160:1585–1591
Jolliet P, Abajo B, Pasquina P, Chevrolet JC (2001) Non-invasive pressure support ventilation in severe community-acquired pneumonia. Intensive Care Med 27:812–821
Fernandez R, Baigorri F, Artigas A (2007) Noninvasive ventilation in patients with “do-not-intubate” orders: medium-term efficacy depends critically on patient selection. Intensive Care Med 33:350–354
Schettino G, Altobelli N, Kacmarek RM (2005) Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 33:1976–1982
Lightowler JV, Wedzicha JA, Elliott MW, Ram FS (2003) Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ 326:185
Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G, Meduri GU (2002) Noninvasive versus conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 28:1701–1707
Keenan SP, Sinuff T, Cook DJ, Hill NS (2003) Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 138:861–870
Acknowledgments
The authors thank all respiratory therapists, nurses and doctors of the medical ICU of National University Hospital, Singapore, for the assistance rendered throughout the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phua, J., Ang, Y.L.E., See, K.C. et al. Noninvasive and invasive ventilation in acute respiratory failure associated with bronchiectasis. Intensive Care Med 36, 638–647 (2010). https://doi.org/10.1007/s00134-009-1743-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1743-6