Abstract
Purpose
To investigate the mechanism of acidosis developing after saline infusion (dilutional acidosis or hyperchloremic acidosis).
Methods
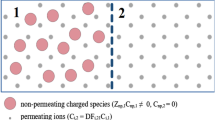
We simulated normal extracellular fluid dilution by infusing distilled water, normal saline and lactated Ringer’s solution. Simulations were performed either in a closed system or in a system open to alveolar gases using software based on the standard laws of mass action and mass conservation. In vitro experiments diluting human plasma were performed to validate the model.
Results
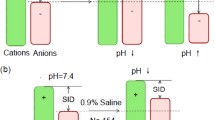
In our computerized model with constant pKs, diluting extracellular fluid modeled as a closed system with distilled water, normal saline or lactated Ringer’s solution is not associated with any pH modification, since all its determinants (strong ion difference, CO2 content and weak acid concentration) decrease at the same degree, maintaining their relative proportions unchanged. Experimental data confirmed the simulation results for normal saline and lactated Ringer’s solution, whereas distilled water dilution caused pH to increase. This is due to the increase of carbonic pK induced by the dramatic decrease of ionic strength. Acidosis developed only when the system was open to gases due to the increased CO2 content, both in its dissociated (bicarbonate) and undissociated form (dissolved CO2).
Conclusions
The increase in proton concentration observed after dilution of the extracellular system derives from the reaction of CO2 hydration, which occurs only when the system is open to the gases. Both Stewart’s approach and the traditional approach may account for these results.



Similar content being viewed by others
References
Kellum JA (1999) Acid–base physiology in the post-Copernican era. Curr Opin Crit Care 5:429–435
Corey HE (2003) Stewart and beyond: new models of acid–base balance. Kidney Int 64:777–787
Siggaard-Andersen O, Fogh-Andersen N (1995) Base excess or buffer base (strong ion difference) as measure of a non-respiratory acid–base disturbance. Acta Anaesthesiol Scand Suppl 107:123–128
Gattinoni L (2009) Foreword. In: Kellum JA, Elbers PWB (eds) Stewart’s textbook of acid–base, 2nd edn. Lulu.com, Amsterdam, pp 21–23
Henderson LJ (1908) The theory of neutrality regulation in the animal organism. Am J Physiol 21:427–448
Siggaard-Andersen O, Engel K (1960) A new acid–base nomogram, an improved method for calculation of the relevant blood acid–base data. Scand J Clin Lab Invest 12:177–186
Grogono AW, Byles PH, Hawke W (1976) An in-vivo representation of acid–base balance. Lancet 1:499–500
Siggaard-Andersen O (1963) Blood acid–base alignment nomogram. Scales for pH, PCO2, base excess of whole blood of different hemoglobin concentrations. Plasma bicarbonate and plasma total CO2. Scand J Clin Lab Invest 15:211–217
Stewart PA (1981) How to understand acid–base. A quantitative acid–base primer for biology and medicine. Elsevier, New York
Kellum JA, Elbers PWB (2009) Stewart’s textbook of acid–base. Lulu.com, Amsterdam
Shires GT, Holman J (1948) Dilutional Acidosis. Ann Intern Med 28:557–559
Asano S, Kato E, Yamauchi M, Ozawa Y, Iwasa M (1966) The mechanism of acidosis caused by infusion of saline solution. Lancet 1:1245–1246
Garella S, Chang BS, Kahn SI (1975) Dilution acidosis and contraction alkalosis: review of a concept. Kidney Int 8:279–283
Kellum JA (2002) Saline-induced hyperchloremic metabolic acidosis. Crit Care Med 30:259–261
Constable PD (2003) Hyperchloremic acidosis: the classic example of strong ion acidosis. Anesth Analg 96:919–922
Hastings AB, Sendroy J Jr (1925) The effect of variation in ionic strength on the apparent first and second dissociation constants of carbonic acid. J Biol Chem 65:445–455
Gattinoni L, Lissoni A (1998) Pathophysiology and diagnosis of respiratory acid–base disturbances in patients with critical illness. In: Bellomo R, Ronco C (eds) Critical care nephrology. Kluwer Academic Publishers, Dordrecht, pp 297–311
(1997) Appendix C: fluids and electrolytes. In: Civetta JM, Taylor RW, Kirby RR (eds) 2257, 3rd edn. Lippincott–Raven, Philadelphia/New York, p 2257
Ring T, Frische S, Nielsen S (2005) Clinical review: renal tubular acidosis–a physicochemical approach. Crit Care 9:573–580
Kellum JA (2000) Determinants of blood pH in health and disease. Crit Care 4:6–14
Scheingraber S, Rehm M, Sehmisch C, Finsterer U (1999) Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 90:1265–1270
Takil A, Eti Z, Irmak P, Yilmaz GF (2002) Early postoperative respiratory acidosis after large intravascular volume infusion of lactated ringer’s solution during major spine surgery. Anesth.Analg 95:294–298
Wilkes NJ, Woolf R, Mutch M, Mallett SV, Peachey T, Stephens R, Mythen MG (2001) The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid–base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 93:811–816
Gattinoni L, Carlesso E, Cadringher P, Caironi P (2006) Strong ion difference in urine: new perspectives in acid–base assessment. Crit Care 10:137
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gattinoni, L., Carlesso, E., Maiocchi, G. et al. Dilutional acidosis: where do the protons come from?. Intensive Care Med 35, 2033–2043 (2009). https://doi.org/10.1007/s00134-009-1653-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1653-7