Abstract
Objective
To compare the colloids 5% albumin, 4% gelatin, and 6% HES 130/0.4 with one another and with normal saline regarding their plasma expanding effects at increased permeability and to compare the results with those from a previous study at normal permeability.
Design and setting
Prospective controlled randomized laboratory study in a university research laboratory.
Subjects
48 adult male Sprague-Dawley rats.
Interventions
Permeability was increased by an injection of 0.5 ml dextran 70 using the fact that dextran causes anaphylactic reaction in the rat. Plasma volume was determined (125I albumin tracer technique) after anesthesia, 1 h after dextran injection (before infusion for 10–15 min of 20 ml/kg bw of each of the colloids or 80 ml/kg saline), and 3 h later. Blood pressure, hematocrit, blood gases, and electrolytes were measured. CVP was measured in four rats.
Measurements and results
Plasma volume was 41.1 ± 1.9 ml/kg at baseline (n = 9), and 29.1 ± 4.1 ml/kg (n = 35) 1 h after the dextran injection. Three hours after infusion of the plasma expander plasma volume had increased by 17.1 ± 3.4 ml/kg in the albumin group, 7.9 ± 3.6 ml/kg in the gelatin group, 7.4 ± 4.4 ml/kg in the HES group, and 12.2 ± 3.1 ml/kg in the saline group. It was unchanged in a control group given no solution (n = 7 for all groups).
Conclusion
Albumin was a more effective plasma volume expander than gelatin or HES or saline (saline in 4 times larger volume). Gelatin and HES were equally effective. All solutions showed a smaller plasma expanding effect than observed in a previous study with normal permeability.
Similar content being viewed by others
Introduction
Increased microvascular permeability is an important pathophysiological alteration in diseases such as sepsis/systemic inflammatory response syndrome and following trauma and results in increased transcapillary leakage of plasma fluid, hypovolemia, and interstitial edema [1, 2, 3]. Hypovolemia decreases cardiac output, resulting in reduced systemic oxygen delivery and generalized vasoconstriction by activation of the baroreceptor reflex by unloading of the high- and low-pressure receptors, which may lead to compromised perfusion in more vulnerable regions such as the gut [4, 5, 6, 7]. Correction of low plasma volume therefore may be essential to maintain adequate organ perfusion and oxygen delivery [8, 9].
Crystalloids (all molecules of molecular weight, MW, less than 30 kDa) and colloids (also containing molecules > 30 kDa) are used as plasma volume expanders. For decades there has been debate regarding whether one should use crystalloids or colloids [10, 11, 12, 13], but there is also a debate regarding the efficacy of different colloids [14, 15, 16]. In contrast to colloids, crystalloids have small effects on coagulation, there is no risk of inducing allergic reactions, and they are inexpensive. Crystalloids are, however, relatively ineffective as plasma volume expanders as they pass freely across the capillary membrane, with fast distribution to the whole extracellular space, and only a minor proportion remaining in the bloodstream [11, 17, 18]. This means that relatively large volumes must be infused to maintain normovolemia with risk of adverse tissue edema [16]. Because of the larger MW the transcapillary passage of colloid solutions is markedly restricted, and they therefore remain in the bloodstream for longer. The oncotic effect of the colloid may also reinforce their plasma expanding capacity [19, 20]. However, the plasma expanding effect of colloids is transient due to a continuous clearance from the circulation related to the rate of degradation, renal and gastrointestinal losses and due to a continuous leakage of macromolecules into the interstitial space [16]. According to the modern two-pore theory of transvascular exchange [21], transcapillary leakage of macromolecules occurs through the large pores of the capillary/venular membrane and is compatible with the view that it is greater at increased permeability than at normal permeability. In addition to size and number of the large pores, transcapillary leakage may also be influenced by charge of the molecules and their interaction with glycocalyx and other endothelial structures [22, 23].
There are still no studies comparing the plasma expanding effect of contemporary colloid solutions or of crystalloids using direct measurements of plasma volume specifically under a condition of increased permeability. The present study was designed to evaluate the plasma expanding effects of 5% albumin, 4% succinylated gelatin, 6% HES 130/0.4, and of saline on rats suffering a generalized increased permeability. The increased permeability was achieved from a bolus injection of dextran, based on the well known phenomenon that dextran induces anaphylactic reaction with increased permeability in the rat [24, 25].
Methods
Materials and anesthesia
The study was approved by the local ethics committee for animal research, and the animals were treated in accordance with the Guidelines of the National Institutes of Health for Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (n = 48) weighing 347 ± 11 g were used. Anesthesia was induced by placing the animal in a covered glass container with a continuous supply of isoflurane (Forene; Abbot, Stockholm, Sweden) and maintained first by inhalation of 1.5–1.8% isoflurane by means of face mask and later by a tracheal cannula after tracheotomy. The animals were placed on a heating pad to maintain a body core temperature (measured rectally) of 37.0–37.3 °C via a feedback circuit. After tracheotomy, the animals were connected to a ventilator (Ugo Basile; Biological Research Apparatus, Comerio, Italy). End-tidal PCO2 was monitored continuously and kept between 4.7 and 5.4 kPa (Capstar-1000, CWE, Ardmore, Pa., USA). The left femoral artery was cannulated for continuous measurement of arterial pressure and to measure arterial blood gases (i-STAT; Hewlett Packard, Böblingen, Germany). The left femoral vein was cannulated and used for injections and infusions. At the end of the experiment the animals were killed by decapitation.
Experimental protocol
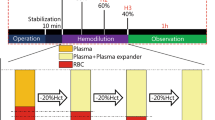
The study was randomized but not blinded and involved four groups with seven rats in each, which were defined according to the volume expander given, and a control group given no solution. Central venous pressure was measured in four separate rats via the right internal jugular vein to evaluate whether venous pressure effects on hydrostatic capillary pressure affect the transcapillary leakage (see “Discussion”). The groups were the control group, the albumin group (5% albumin, Aventis Behring, Marburg, Germany), the gelatin group (4% gelofusine, Braun, Melsungen, Germany), the HES group (6% HES 130/0.4, Voluven, Fresenius, Halden, Norway), and the saline group (0.9% NaCl, Fresenius, Halden, Norway). Following a stabilization period of 15 min after tracheotomy and vascular cannulations the animals received an intravenous injection of 0.5 ml dextran 70 (Macrodex 6%; Pharmalink, Upplands Väsby, Sweden) for the purpose of increasing microvascular permeability.
The colloid solutions at a dose of 20 ml/kg body weight or normal saline at a dose of 80 ml/kg were given 1 h after the dextran injection (Fig. 1). Unpublished observations from previous experiments have shown that the decrease in plasma volume following a dextran injection reaches its maximum within 1 h, a result confirmed in the present study in the control group given no plasma expander (see “Results”). The time for infusion was 10 min for the colloids but 15 min for saline to minimize the risk for acute fluid overload. Arterial blood gases were measured just before the dextran injection (baseline values), 1 h after injection of dextran before infusion of the plasma expander, immediately after the infusion, and 3 h after the infusion (Fig. 1). Plasma baseline volume was measured after finishing the preparation, 1 h after the dextran injection before the infusion, and 3 h after the infusion (Fig. 1).
The plasma volume (V) was calculated by measurement of the increase in radioactivity per milliliter of plasma (ΔC2) after an intravenous injection of a known amount of activity of 125I-labeled albumin (C1) (31): V = C1/ΔC2. Radioactivity was measured by gamma counter (Wizard 1480, LKB-Wallace, Turku, Finland). The increase in radioactivity of 125I-labeled albumin (ΔC2) was determined by subtracting the activity in a blood sample taken before the injection from that taken 5 min after the injection, thereby taking into account the cumulative effects of radioactivity. The blood was centrifuged and the radioactivity in a fixed volume of plasma was determined. To determine the exact dose injected, the radioactivity of the emptied vial, the syringe and the needles used was subtracted from the total radioactivity in the prepared dose.
Statistics
Results are presented as mean values ± SD. Statistical comparisons between groups were performed with the nonparametric Mann-Whitney rank sum test and analysis of variance which, when necessary, was adjusted for multiple comparisons (Bonferroni). Differences with p values less than 0.05 were considered statistically significant. Sigma Stat 2.0 software was used for statistical analysis.
Results
Physiological data
Data on hemoglobin (Hb), sodium (Na+), potassium (K+), pH, base excess (BE), PaO2, and PaCO2 in the different groups are summarized in Table 1. Hb increased from 135 ± 6 before the dextran infusion to 157 ± 13 g/l (n = 35) 1 h after the dextran infusion (p < 0.05). Infusion of the plasma expander caused a decrease in Hb, followed by a subsequent increase. At the end of the experiment Hb levels had increased in all groups, reaching significance compared with the baseline values in the gelatin and HES groups. Na+ and K+ did not differ between groups. Mean arterial blood pressure values at baseline, 1 h after the dextran injection, after infusion of the plasma volume expander, and 3 h later at the end of the experiment are presented in Table 2. There was a lower arterial blood pressure 1 h after the dextran injection and 3 h after infusion of the plasma expander than at baseline. Arterial blood pressure was lower for all solutions at end of the experiment than shortly after the infusion. For pH, PaO2, and PaCO2, and BE there was no difference between the groups except from a decrease in BE in the saline group after the saline infusion and at the end of the experiment. In the four experiments performed for measurement of central venous pressure baseline central venous pressure was 3.0 ± 0.3 mmHg. It was 2.2 ± 0.7 mmHg 1 h after the dextran injection, 3.3 ± 0.5 mmHg after the albumin infusion, and 3.0 ± 0.8 at end of the experiment.
Plasma volume
Baseline plasma volume determined in separate experiments (n = 9) was 41.1 ± 1.9 ml/kg. Plasma volume directly before infusion of the plasma volume expander was 29.1 ± 4.1 ml/kg (n = 35; p < 0.05) with no difference between the five groups. The remaining increase in plasma volume 3 h after infusion of plasma expander (20 ml/kg for the colloids and 80 ml/kg for saline; (PV3 - PV2 in Fig. 1) is shown in Fig. 2; it was 17.1 ± 3.4 ml/kg in the albumin group, 7.9 ± 3.6 ml/kg in the gelatin group, 7.4 ± 4.4 ml/kg in the HES group, and 12.2 ± 3.1 ml/kg in the saline group. The plasma expanding effect was larger in the albumin group than in the other groups (p < 0.05). There were no significant differences between the HES, the gelatin, and the saline groups. There was no change in plasma volume in the control group from 1 h after the dextran injection to the end of the experiment.
Increase in plasma volume (ΔPV: PV3 – PV2 in Fig. 1) 3 h after the bolus infusion of 20 ml/kg for colloids and 80 ml/kg for saline (0.9% NaCl), compared to the plasma volume 1 h after dextran injection, in the five groups analyzed. It can be seen that the albumin group had a better volume expanding effect than the other groups. There was no significant difference between the gelatin, HES, and saline groups. The plasma volume did not change in the control group. *p < 0.05
Discussion
The present study in rats was designed to compare clinically available plasma expanders regarding their capacity to restore reduced intravascular volume during a state of increased microvascular permeability. The permeability was increased in a standardized manner by inducing anaphylactic reaction with a small fixed bolus injection of dextran. Within 1 hour after the dextran injection the anaphylactic reaction resulted in a reduction in plasma volume from baseline values of about 41 ml/kg to about 29 ml/kg. There was a simultaneous increase in Hb concentration and decrease in arterial blood pressure. The results show that, under the present experimental circumstances when the colloids are given in equal volumes and normal saline in a four times greater volume, 5% albumin is a more effective plasma volume expander than 4% gelatin or 6% HES130/0.4 or saline. Gelatin and HES were equally effective. The finding of a better plasma expanding effect of albumin than that of HES and gelatin is compatible with the observations that hemoglobin concentration was lower in the albumin group than in the HES and gelatin groups at end of the experiments (Table 1).
The well known fact that dextran induces an anaphylactic reaction in the rat [24] was used in the present study to induce a standardized increase in permeability. Increased permeability with transcapillary leakage was confirmed by the visually observed marked peripheral edema that developed shortly after the dextran injection by the equally large reduction in plasma volume for all groups, by the reduction in mean arterial pressure (Table 2), and by the increase in hemoglobin concentration (Table 1).
The tracer albumin technique is well established for measurement of plasma volume. Because of transcapillary escape of albumin during the 5-min period between tracer injection and blood sampling, the albumin-derived radioactivity measured in plasma may have been somewhat decreased, resulting in overestimation of the plasma volume. The overestimation, however, must be equally large for all groups and it must be small as the blood sample was taken very shortly (5 min) after the tracer injection. The fact that measured baseline plasma volume of about 41 ml/kg are in the same range as those presented in the literature for rats of 39–42 ml/kg [26] confirms the reliability of the plasma volume measuring technique used.
An increase in transcapillary leakage of macromolecules at increased permeability is compatible with the two-pore theory of transcapillary fluid exchange [21]. According to this theory, fluid and smaller solutes pass the capillary membrane through all pores along the entire microvascular bed, whereas macromolecules pass the capillary membrane only through the 10–30 × 103 times less common larger pores present in venules and at the venous side of the capillary network. The transcapillary/transvenular hydrostatic pressure is the only force responsible for fluid flow through the large pores, as the oncotic pressure across these pores is virtually zero [21]. This means that the macromolecules are lost to the interstitium mainly through convection by following the large-pore fluid flux, while the diffusion force is of less importance [21]. This hypothesis means that there is always a continuous leakage of macromolecules (normal transcapillary escape rate) and even a minute increase in total pore area via the large pores may cause a substantially greater loss of macromolecules. This means that the plasma expansion of a colloid must be less effective at increased permeability than at normal permeability. Thus the two-pore theory is compatible with a less effective plasma expanding effect of colloids at raised than at normal permeability.
This hypothesis also means that the leakage of proteins through the large pores increases with an increase in hydrostatic capillary pressure. If so, the leakage is higher at a high systemic arterial pressure than at a low one. Transcapillary leakage of proteins can therefore be expected to increase after volume expansion, if the infusion is followed by an increase in arterial pressure. Our results that there was no loss of plasma volume in the control group from 1 h after the dextran infusion until end of the experiment, while there was a significant loss of plasma in the groups given a plasma expander, may be explained by the lower arterial pressure in the control group (Table 2). The hypothesis also means that avoidance of supranormal arterial pressure may help to preserve the plasma volume and to reduce the need for plasma expanders.
The plasma volume expanders analyzed in the present study have been examined in the rat previously in our laboratory but under the condition of normal permeability [27]. In that study blood volume was reduced before the infusion by a standardized hemorrhage of 16 ml/kg, a reduction in plasma volume of about the same magnitude as that obtained by the dextran infusion in the present study. While plasma volume increased by 21.1 ml/kg 3 h after the infusion of 20 ml/kg 5% albumin in our previous study, it increased by 17.1 ml/kg in the present study. Corresponding values for gelatin were 13.1 vs. 7.9 ml/kg, and 13.8 vs. 7.4 ml/kg for HES 130/0.4. For saline, after the infusion of 80 ml/kg the values were 16.0 ml/kg in the previous study vs. 12.2 ml/kg. This difference in plasma loss between the two studies may, if anything, even have been somewhat underestimated due to a larger leakage of tracer albumin during the 5-min period between injection and blood sampling when permeability is increased.
In addition, arterial blood pressure was lower in the present study than in our previous study, most likely an effect of the dextran-induced anaphylactic reaction. Thus the blood pressure in the present study was 5–10 mmHg lower after infusion of the plasma expander and 15–20 mmHg lower at end of the experiments (Table 2) [27]. If blood pressure is a factor influencing transcapillary fluid loss as discussed above, the lower blood pressure have saved plasma in the present study compared to the previous study [27]. Thus also this factor means that the effect of permeability on plasma volume leakage, if anything, is underrated when making a direct comparison between the two studies.
If, as discussed, the hydrostatic capillary pressure is of importance for transcapillary fluid loss, a change in venous pressure may also have influenced the hydrostatic pressure. Differences in venous pressure, however, cannot explain the difference in plasma expansion between the groups as the better the plasma expansion of the solution the higher the venous pressure. The fact that central venous pressures measured in four separate rats given the best plasma expander albumin did not increase to values above normal (see “Results”) also indicate that a difference in venous pressure cannot explain the difference between plasma expansion in the present and the previous study [27]. Nor can the difference in Hb explain the difference in plasma expansion between the two studies, as experimental studies have shown that plasma leakage is lower at a high than at a lowered Hb [28], and the Hb values were higher in the present study than in our previous study. Taking all these arguments together, comparison of the two studies thus strongly indicates that the plasma expanding effect of all solutions analyzed is lower at increased than at normal permeability.
It is well accepted, and also in line with the two-pore theory [21], that crystalloids are rapidly distributed to the entire extracellular space, and that the volumes infused must be much larger than those for colloids for the same plasma expanding effect. This hypothesis was confirmed in the present study in which the plasma expanding effect of saline was worse than that with albumin and showed only a tendency (not significant) of better volume effect than with gelatin and HES when saline was given in a four times larger volume (Fig. 2).
The difference in plasma expanding effects of the colloids analyzed may have several explanations. Factors of importance may be average MW, MW distribution, oncotic pressure of the solution, degradation rate, threshold for renal elimination, molecular shape, electrical charge, and interference with glycocalyx [17, 18, 20, 22, 23, 29, 30]. Gelatin and HES are neutral molecules whereas albumin is negatively charged, which may prevent its penetration. Albumin is a monodisperse solution with a MW of 69 kDa for all molecules, with insignificant degradation rate. Both HES and gelatin are polydisperse solutions with molecules ranging from very small to very large, and the larger molecules undergo enzymatic degradation to smaller molecules. The small molecules disappear relatively quickly from the intravascular space to the interstitium and are cleared through the kidneys. HES solutions, and especially HES 130/0.4 with its low degree of substitution, are degraded by amylase to smaller molecules. The degradation rate of HES may be faster in rats than in humans, due to the higher amylase concentration in rats. The gelatin molecules, with a relatively small average MW of 35 kDa, undergo degradation by proteases in the reticuloendothelial system. Thus several factors may be responsible for the better plasma volume expanding effect with 5% albumin than with gelatin and HES in the present study, and why gelatin and HES were equally effective despite the difference in MW.
In conclusion, the present study on rats with increased permeability shows that during a 3-h period the treatment of hypovolemia with 5% albumin preserves plasma volume better than with 4% gelatin and 6% HES 130/0.4, or with normal saline given in a four times larger volume. Gelatin and HES are equally effective. Comparison of these results with those from a previous study on rats with normal permeability indicates that the plasma expanding effect of these solutions is less effective when permeability is increased.
References
Groeneveld AB, Teule GJ, Bronsveld W, van den Bos GC, Thijs LG (1987) Increased systemic microvascular albumin flux in septic shock. Intensive Care Med 13:140–142
Christ F, Gamble J, Garside IB, Kox WJ (1998) Increased microvascular water permeability in patients with septic shock, assessed with venous congestion pletismography (VCP). Intensive Care Med 24:18–27
Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledningham IM, Calman KC (1985) Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet I:781–784
Hinshaw LB (1996) Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med 24:1072–1078
Hollbeck S, Grände PO (2002) Hypovolemia is a main factor behind disturbed perfusion and metabolism in the intestine during endotoxaemia in cat. Shock 18:367–373
Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA (2001) Postoperative multiple organ failure: the role of the gut. Shock 15:1–10
Fink MP (1991) Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med 19:627–641
Imm A, Carlson RW (1993) Fluid resuscitation in circulatory shock. Crit Care Clin 9:313–933
Haupt MT, Gilbert EM, Carlson RW (1985) Fluid loading increases oxygen consumption in septic patients with lactic acidosis. Am Rev Respir Dis 131:912–916
Virgilio RW, Rice CL, Smith DE, James DR, Zarins CK, Hobelmann CF, Peters RM (1979) Crystalloid vs. colloid: is one better? A randomised clinical study. Surgery 105:65–71
Hillmann K, Bishop G, Bristow P (1997) The crystalloid versus colloid controversy: present status. In: Haljamae H (ed) Plasma volume support. Baillière Tindall, London, pp 1–13
Roberts I, Alderson P, Bunn F, Chinnock, Ker K, Schiethout G (2004) Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev CD000567
Schierhout G, Roberts I (1998) Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systemic review of randomised trials. BMJ 316:961–964
Beards SC, Watt T, Edwards JD, Nightingale P, Farragher EB (1994) Comparison of the hemodynamic and oxygen transport responses to modified fluid gelatine and hetastarch in critically ill patients: a prospective, randomised trial. Crit Care Med 22:600–605
Marx G, Cobas Mayer M, Schuerholz T, Vangerow B, Gratz K, Hecker H, Sumpelmann R, Rueckoldt H, Leuwer M (2002) Hydroxyethyl starch and modified fluid gelatine maintain plasma volume in a porcine model of septic shock with capillary leakage. Intensive Care Med 28:629–635
Marx G (2003) Fluid therapy in sepsis with capillary leakage. Eur J Anaesthesiol 20:429–442
Haljamae H (1985) Rationale for the use of Colloids in the treatment of shock and hypovolaemia. Acta Anaesthesiol Scand Suppl 82:48–54
McIlroy DR, Kharasch ED (2003) Acute intravascular volume expansion with rapidly administered crystalloid or colloid in the setting of moderate hypovolemia. Anesth Analg 96:1572–1577
Grocott M, Mythen M (1999) Fluid therapy. In: Baillière's clinical anaesthesiology. Harcourt. pp 363–381
Vercueil A, Grocott M, Mythen MG (2005) Physiology, pharmacology, and rationale for colloid administration for the maintenanace of effective hemodynamic stability in critically ill patients. Transfus Med Rev 19:93–109
Rippe B, Haraldsson B (1994) Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 74:163–219
Grocott MP, Hamilton MA (2002) Resuscitation fluids. Vox Sang 82:1–8
Bold J, Suttner S (2005) Plasma substitutes. Minerva Anestesiol 71:741–758
Guo Y, Hedquist P, Gustafsson LE (2001) Absence of mast cell involvement in active systemic anaphylaxis in rats. Eur J Pharmacol 430:305–310
Majde JA (2003) Animal models for hemorrhage and resuscitation research. J Trauma 54:S100–S105
Lundin S, Folkow B, Rippe B (1981) Central blood volume in spontaneously hypertensive rats and Wistar-Kyoto normotensive rats. Acta Physiol Scand 112:257–262
Persson J, Grände PO (2005) Volume expansion of albumin, gelatin, hydroxyethyl starch, saline and erythrocytes after haemorrhage in the rat. Intensive Care Med 31:296–301
Valeri CR, Cooper AG, Pivacek LE (1973) Limitations of measuring blood volume with iodinated I 125 serum albumin. Arch Intern Med 132:534–538
Arfors K-E, Buckley PB (1997) Pharmacological characteristics of artificial colloids. In: Haljamae H (ed) Plasma volume support. Saunders, London, pp15–47
Taylor AE, Granger DN (1984) Exchange of macromolecules across the microcirculation. In: Handbook of physiology. American Physiology Society, Bethesda
Acknowledgements
This study was supported by grants from the Swedish Research Council (#11581) and from the Medical Faculty of Lund University, Sweden. We are grateful to Helén Davidsson for highly skilled technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubniks, M., Persson, J. & Grände, PO. Plasma volume expansion of 5% albumin, 4% gelatin, 6% HES 130/0.4, and normal saline under increased microvascular permeability in the rat. Intensive Care Med 33, 293–299 (2007). https://doi.org/10.1007/s00134-006-0454-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0454-5