Abstract
Objective
To compare the causative pathogens of early-onset and late-onset ventilator-associated pneumonia (VAP) diagnosed by bronchoalveolar lavage quantitative cultures. Most previous reports have been based on endotracheal aspirate cultures and gave uncertain findings.
Design
Prospective evaluation of consecutive patients with clinical suspicion for VAP.
Setting
Multidisciplinary intensive care unit of a university hospital.
Patients and participants
During a 3-year period 473 patients with clinical suspicion of VAP entered the study. Diagnosis of VAP was confirmed by cultures of bronchoalveolar lavage (>104 cfu/ml) specimens in 408 patients.
Interventions
Protected bronchoalveolar lavage samples were taken. Initial antibiotic therapy was modified upon bronchoalveolar lavage culture results.
Measurements and results
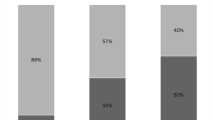
Among 408 patients 191 had early-onset (<7 days mechanical ventilation) and 217 late-onset (≥7 days) VAP. Potentially multiresistant bacteria, mainly Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA), were the most commonly isolated pathogens in both types of VAP. No difference was noted in the contribution of potentially multiresistant pathogens (79% vs. 85%), P. aeruginosa (42% vs. 47%), or MRSA (33% vs. 30%) between early-onset and late-onset VAP. Initial antibiotic therapy was modified in 58% of early-onset VAP episodes and in 36% of late-onset VAP episodes. No difference in mortality was found between the two types of VAP.
Conclusions
Both early-onset and late-onset VAP were mainly caused by potentially multiresistant bacteria, most commonly P. aeruginosa and MRSA. Antimicrobial agents against these pathogens should be prescribed empirically, at least in our institution.
Similar content being viewed by others
Introduction
The initial, empirical antibiotic therapy of ventilator-associated pneumonia (VAP) is often based on timing of its occurrence in relation to the onset of mechanical ventilation [1]. This is due to reported differences between causal pathogens associated with early-onset (<5–7 days mechanical ventilation) compared to late-onset (≥5–7 days mechanical ventilation) VAP [2, 3]. Early-onset VAP is most often caused by core micro-organisms (e.g., Streptoccoccus pneumoniae, enteric Gram-negative bacilli, methicillin-susceptible Staphylococcus aureus), whereas late-onset VAP is most commonly due to potentially multiresistant bacteria (e.g., Pseudomonas aeruginosa, Acinetobacter baumanni, methicilline resistant S. aureus) [3, 4, 5, 6]. However, Ibrahim et al. [7] reported that both early-onset and late-onset VAP may be associated with similar, usually multiresistant, pathogens. Nevertheless, most of the above reports were based on studying patients with VAP diagnosed on the basis of clinical criteria and endotracheal aspirate cultures, which make their conclusions uncertain [8].
Therefore the aim of our study was to compare the causative pathogens of early-onset and late-onset VAP diagnosed by bronchoalveolar lavage (BAL) quantitative cultures, because BAL samples are considered to give more accurate results than endotracheal aspirates [9, 10, 11, 12].
Methods
Patients
The study was conducted at the University of Thrace teaching hospital (500 beds) during a 3-year period (September 2000–September 2003) and was approved by the Human Studies Ethics Committee. All patients admitted to the multidisciplinary intensive care unit (15 beds) were potentially eligible for this investigation. Inclusion criteria were: age over 18 years, at least 48 h of mechanical ventilation, and clinical suspicion of VAP [3, 7, 12], defined by new and persistent (present for >72 h) infiltrate on chest radiography, plus two of the following items: (a) purulent tracheal secretions (>25 neutrophils per high power field using Gram’s stain), (b) body temperature above 38.3°C or below 36°C, and (c) leukocytosis (>10,000 cells/µl) or leukopenia (<5,000 cells/µl). Patients were excluded if (a) they were temporarily transferred to our intensive care unit due to lack of available bed in another hospital, (b) they had received immunosuppressants or long-term corticosteroid therapy (>0.5 mg/kg prednisolone/day for >1 month) during the previous year, (c) they had neutropenia (leukocyte count <1,000/µl or neutrophil count <500/µl), or (d) they had concomitant acquired immune-deficiency syndrome.
Of the 1,467 patients admitted to the intensive care unit during the study period 1,059 (72%) required mechanical ventilation longer than 48 h, and 535 of these (51%) had clinical suspicion of VAP. Sixty-two (12%) were excluded (28 had received immunosuppressants or long-term corticosteroid therapy, 8 were temporarily transferred to our intensive care unit, 3 had acquired immune deficiency syndrome, and 23 had neutropenia). Therefore 473 patients with clinical suspicion of VAP were evaluated with BAL. Diagnosis of VAP was finally confirmed by quantitative cultures of BAL in 408 (86%), and these patients represented the study population.
Study design, data collection, and end-points
In patients with clinical suspicion for VAP in daily rounds, protected BAL samples were taken by fiberoptic bronchoscopy, as previously described [13]. BAL specimens were cytocentrifuged and divided into two preparations, one for Gram stain and the other for quantitative culture. The culture results were available within 2 days. The diagnosis of VAP was confirmed if at least one bacterial species grew at a concentration above the predetermined threshold (>104 cfu/ml) [6]. BAL specimens were always obtained before introduction of any antibiotics. Initial antibiotic therapy was modified in the light of quantitative BAL culture results when at least one cultured isolate proved resistant in vitro to the administered regimen or combination therapy was necessary because of isolation of P. aeruginosa [8]. Deescalation of the initial regimen for a narrower spectrum alternative after BAL results was not used. The treatment regimen that replaced the initial one consisted of at least one antibiotic to which all isolates were susceptible in vitro. In the presence of P. aeruginosa at least two active agents (combination therapy) were used [8]. Quantitative BAL cultures yielding less than significant growth of organisms were not taken into account in order to modify the initial antibiotic therapy; nevertheless these patients were not included as only patients with bacterial species growth greater than 104 cfu/ml in BAL cultures were studied. Only the first VAP episode of each patient was recorded. No patient received oropharyngeal or selective digestive decontamination. Histamine type 2 receptors antagonists were used in all patients to prevent upper digestive tract hemorrhage.
At the time of study entry and before bronchoscopy we recorded each patient’s age, sex, admission diagnostic category (medical vs. surgical, trauma vs. nontrauma surgical), concomitant diseases, indication for mechanical ventilation, severity of illness based on Acute Physiology and Chronic Health Evaluation (APACHE) II [14], and severity of organ dysfunction based on Sequential Organ Failure Assessment (SOFA) [15]. The presence of potential specific risk factors for development of VAP were also recorded and included the administration of histamine type 2 receptor antagonists, antacids, sucralfate, corticosteroids, or vasopressors, tracheostomy, dialysis, reintubation, duration of mechanical ventilation, and previous 2 weeks use of antibiotics. The initial, empirical antibiotic regimen was always chosen by the attending physicians, usually based on American Thoracic Society recommendations [3].
We compared the findings in patients with early-onset VAP (<7 days mechanical ventilation) with those in patients with late-onset VAP (≥7 days mechanical ventilation). We used a cutoff of 7 days because it corresponded to the median time of occurrence of VAP in the 408 patients studied, and because mechanical ventilation lasting 7 days or more is associated with increased likelihood of infection with a potentially multiresistant mico-organism [2, 8]. Among 408 patients 191 (47%) had early-onset and 217 (53%) late-onset VAP.
The primary end-point was comparison of causative pathogens between early-onset and late-onset VAP. Secondary end-points were the comparison of frequency of initial antibiotic treatment modification, and 15-day, 28-day, intensive care unit, and hospital mortality between early-onset and late-onset VAP.
Statistics
Statistical analysis was performed using Norusis MJ SPSS version 11.0 (SPSS, USA). The χ2 test with Yates’ correction was used to compare categorical variables. Continuous variables, normally or abnormally distributed, were compared using Student’s t test or Wilcoxon’s signed rank test, respectively. Differences with a p value less than 0.05 were considered statistically significant.
Results
Characteristics of patients with early-onset and late-onset VAP at study entry are presented in Table 1. With the exception of vasopressors use and tracheostomy, which were more frequent in late-onset VAP, other characteristics did not differ significantly between the two groups.
Pathogens identified and initial treatment modifications
Micro-organisms responsible for VAP are shown in Table 2. Potentially multiresistant bacteria, mainly P. aeruginosa and MRSA, were the most commonly isolated pathogens in both early-onset and late-onset VAP. No significant difference was noted in the contribution of potentially multiresistant pathogens between early-onset and late-onset VAP (79% vs. 85%, p=0.06). This was also the case for P. aeruginosa, the most common Gram-negative pathogen (42% vs. 47%, p=0.26) and for MRSA, the most common Gram-positive pathogen (33% vs. 30%, p=0.39). Characteristics of patients with early-onset VAP caused either by P. aeruginosa/MRSA or by other pathogens at study entry are presented in Table 3. Risk factors of early-onset VAP due to P. aeruginosa or MRSA were previous use of antibiotics, corticosteroid therapy, vasopressors use, trauma, and neurological emergency.
Among 408 VAP episodes 236 (58%) were monomicrobial (one bacterial species in BAL) and 172 (42%) polymicrobial (two or more bacterial species in BAL) in origin. Monomicrobial were 124 of 191 episodes of early-onset VAP (65%) and 112 of 217 episodes of late-onset VAP (52%); polymicrobial were the remaining 67 episodes of early-onset VAP (35%) and 105 episodes of late-onset VAP (48%; p=0.008).
After BAL culture results, initial antibiotic therapy was modified in 189 of 408 VAP episodes (46%): in 111 of 191 episodes of early-onset VAP (58%) and in 78 of 217 episodes of late-onset VAP (36%; p<0.001). Strains responsible for this modification in early-onset and late-onset VAP are presented in Table 4. In early-onset VAP initial antibiotic regimen modification was due to isolation of P. aeruginosa in 76 VAP episodes (69%), MRSA in 27 VAP episodes (24%), and both P. aeruginosa and MRSA in the remaining 8 of 111 VAP episodes (7%). Among 84 P. aeruginosa strains 36 (43%) were resistant to the initial regimen, and 48 (57%) were sensitive but modification of initial therapy was required due to addition of a second effective agent. In late-onset VAP initial antibiotic treatment modification was due to isolation of P. aeruginosa in 38 VAP episodes (49%), MRSA in 30 (38%), and both P. aeruginosa and MRSA in the remaining 10 of 78 VAP episodes (13%). In contrast to early-onset, in late-onset VAP all strains of P. aeruginosa responsible for treatment modification were resistant to the initial regimen.
Antibiotic therapy used at pre-BAL and post-BAL periods, and susceptibility to antibiotics of cultured pathogen strains in early-onset and late-onset VAP are shown in Tables 5 and 6, respectively.
Mortality rate
Crude intensive care unit and hospital mortality of patients with VAP were 13% and 16%, respectively. No significant difference was found in 15-day, 28-day, intensive care unit, or hospital mortality between early-onset and late-onset VAP (Table 1). Hospital mortality was higher in early-onset VAP caused by P. aeruginosa and/or MRSA than in early-onset VAP caused by other pathogens (Table 3).
Discussion
The main finding of the present study, which confirmed the responsible pathogens of VAP by BAL quantitative cultures, was that both early-onset and late-onset VAP cases are mainly caused by potentially multiresistant micro-organisms, most commonly P. aeruginosa and MRSA. As a result, initial antibiotic therapy was modified more frequently in early-onset than in late-onset VAP because attending physicians did not expect such high prevalence of potentially multiresistant organisms in early-onset VAP.
Several studies have shown that early-onset and late-onset VAP are caused by different pathogens [2, 3, 4, 5, 6]. In contrast, Ibrahim et al. [7] reported that pathogens associated with early-onset and late-onset VAP may be similar and frequently multiresistant; however, because microbiological diagnosis of VAP in this study was usually made on the basis of endotracheal aspirate cultures, which were not always quantitative, its findings were considered uncertain [8]. The present study confirms by BAL quantitative cultures for the first time, to our knowledge, that early-onset and late-onset VAP episodes are caused by similar pathogens, which are usually multiresistant.
The importance of our findings is that they may influence antimicrobial prescribing practices in the intensive care unit. Indeed, these findings suggest that antimicrobial agents against P. aeruginosa and MRSA should be prescribed empirically, at least in our institution, to patients suspected of having either early-onset or late-onset VAP. This may help to reduce the occurrence of inadequate or ineffective antimicrobial therapy, which has been associated with poorer patient outcomes [16, 17, 18]. According to the American Thoracic Society consensus statement [3], early-onset VAP is usually due to core pathogens, and monotherapy is recommended. Highly resistant P. aeruginosa and MRSA are not included among these core bacteria. Therefore initial antibiotic therapy prescribed by the attending physicians, usually based on the American Thoracic Society recommendations, resulted in undertreating of patients with early-onset VAP of the present study, and therapy modification was required in 58% of cases. In patients who develop late-onset VAP the most commonly encountered pathogens are potentially multiresistant Gram-negative bacteria, including P. aeruginosa and Acinetobacter species as well as MRSA, and the American Thoracic Society recommendations for the empirical treatment of these patients include the use of combination antimicrobial therapy with drugs that are active against P. aeruginosa and vancomycin for severely ill patients with suspected MRSA infection [3]. Despite prescription of rather adequate combination antibiotic therapy in most patients with late-onset VAP of our study, initial therapy modification was required in 36% of VAP episodes because of frequent isolation of resistant P. aeruginosa strains or not inclusion in the initial regimen of drugs active against MRSA (Table 4).
Several studies [19, 20, 21, 22] have demonstrated that the success rate of empirical monotherapy in early-onset VAP is similar to that of combination therapy, and this is probably the case in patients who have not received antibiotics previously [2, 8]. However, the findings of the present study as well as those of others [7] suggest that combination antipseudomonal therapy and vancomycin are initially required in early-onset VAP.
The high rate of early-onset VAP due to potentially multiresistant bacteria in this study may be due in part to the prior use of antibiotics; over 99% of patients with early-onset VAP caused by P. aeruginosa and/or MRSA received antimicrobial therapy prior to the development of this infection (Table 3). Previous investigations have shown a strong association between prior antibiotic use and the subsequent development of VAP, particularly VAP caused by potentially antibiotic-resistant pathogens [2, 7, 23, 24, 25, 26, 27]. Another factor potentially contributing to the explanation of our findings in patients with early-onset VAP is frequent short-term corticosteroid use (Table 3). In fact, corticosteroids predispose to VAP with P. aeruginosa [3].
Our findings of similar mortality rates in early-onset and late-onset VAP (Table 1) are consistent with those reported by other investigators in different countries [7, 28, 29]. One potential explanation suggested by the results of the present and other [7] studies is that patients with either early-onset or late-onset VAP have similar rates of infection with high-risk multiresistant pathogens, mainly P. aeruginosa and MRSA, which are associated with higher rates of attributable hospital mortality [24]. Hospital mortality in the present study was higher in early-onset VAP caused by P. aeruginosa and/or MRSA than in early-onset VAP caused by other pathogens (Table 3).
The present study has the advantage of using protected BAL quantitative cultures to identify the causative pathogens in a large sample size of VAP. Although we are aware of the potential limitations of this method [11, 30, 31, 32], it is currently considered one of the best invasive techniques for the microbiological diagnosis of VAP [9, 10, 11, 12]. However, the findings of this study may not be applicable to other intensive care units with lower rates of VAP caused by P. aeruginosa and MRSA. That is because our patient population may not be similar to those of other intensive care units. The high incidence of prior exposure to antibiotics among our patients may not be representative of practices at other institutions. Significant variations in the bacterial pathogens associated with VAP have been found between four European intensive care units [33]; multiresistant bacteria associated with early-onset VAP were isolated in two of these, thus supporting the existence of variability between intensive care units of different hospitals in terms of the causal agents of VAP.
In conclusion, the present study demonstrates that in our intensive care unit both early-onset and late-onset VAP were caused mainly by potentially multiresistant bacterial pathogens, most commonly P. aeruginosa and MRSA. Consequently, the empirical antibiotic therapy prescribed was inadequate in a significant proportion of patients, and therapy modification was required. These findings, rather than providing information generally applicable, emphasize the need of intensive care unit-specific knowledge of causal agents associated with VAP [34]; this information can influence local antibiotic prescribing practices and reduce the rate of administration of inadequate antimicrobial therapy.
References
Wunderink RG (1997) Therapy for nosokomial pneumonia. Curr Opin Pulm Med 3:120–124
Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, Gibert C (1998) Ventilator associated pneumonia caused by potentially drug resistant bacteria. Am J Crit Care 157:531–539
American Thoracic Society (1996) Hospital acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy and preventive strategies. Am J Respir Crit Care 153:1711–1725
Baker AM, Meredith JW, Haponik EF (1996) Pneumonia in intubated trauma patients: microbiology and outcomes. Am J Crit Care 153:343–349
Niederman MS, Craven DE, Fein AM, Schultz DE (1990) Pneumonia in the critically ill hospitalized patients. Chest 97:170–181
Rello J, Ricard M, Ausina V, Net A, Prats G (1992) Pneumonia due to Haemophilus influenzae among mechanically ventilated patients: incidence, outcome and risk factors. Chest 102:1562–1565
Ibrahim EH, Ward S, Sherman G, Kollef M (2000) A comparative analysis of patients with early vs late onset nosokomial pneumonia in ICU setting. Chest 117:1434–1442
Rello J, Paiva JA, Bairabar J, Barcenilla F, Bodi M, Castander D, Correa H, Diaz E, Garnacho J, Llorio M, Rios M, Rodriguez A, Sole-Violan J (2001) International conference for the development of consensus on the diagnosis and treatment of ventilator associated pneumonia. Chest 120:955–970
Chastre J, Fagon J (1994) Invasive diagnostic testing should be routinely used to manage ventilated patients with suspected pneumonia. Am J Respir Crit Care Med 150:570–574
Sanchez-Nieto JM, Torres A, Garcia-Cordoba F, El-Ebiary M, Carrillo A, Ruiz J, Nunez ML, Niederman M (1998) Impact of invasive and noninvasive quantitative cultures sampling on outcome of ventilator associated pneumonia. Am J Respir Crit Care Med 157:371–376
Torres A, El-Ebiary M, Padro L, Gonzalez J, de la Bellacasa JP, Ramirez J, Xaubet A, Ferrer M, Rodriguez-Roisin R (1994) Validation of different techniques for the the diagnosis of ventilator associated pneumonia. Am J Respir Crit Care Med149:324–331
Fagon JY, Chastre J, Wolff M, Gervais C, Parer-Aubas S, Stephan F, Similowski T, Mercat A, Diehl JL, Sollet JP, Tenaillon A (2000) Invasive and noninvasive strategies for the management of suspected ventilator associated pneumonia: a randomized trial: the VAP group. Ann Intern Med 132:621–630
Meduri GU, Beals DH, Maijub AG, Baselski V (1991) Protected bronchoalveolar lavage: a new bronchoscopic technique to retrieve uncontaminated distal airway secretions. Am Rev Respir Dis 143:855–864
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A (1991) The APACHE II prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619–1636
Vincent JL, De Mondonka A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of SOFA to assess the incidence of organ dysfunction/failure in intensive care units: results of multicenter prospective study. Crit Care Med 1998 26:1793–1800
Luna C, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly E (1997) Impact of BAL data on the therapy and outcome of ventilator associated pneumonia. Chest 111:676–685
Rello J, Gallego M, Mariscal D, Sonora R, Valles J (1997) The value of routine microbial investigation in ventilator associated pneumonia. Am J Respir Crit Care 156:196–200
Kollef MH, Sherman G, Ward S, Fraser VJ (1999) Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474
Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ (1993) Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 37:1073–1081
Mangi RJ, Greco T, Ryan J, Thornton G, Andriole VT (1988) Cefoperazone versus combination antibiotic therapy of hospital acquired infection. Am J Med 84:68–74
Schentag JJ, Vari AJ, Winsdale NE, Swanson DJ, Smith IL, Simons GW, Vigano A (1985) Treatment with aztreonam or tobramycin in critical care patients with nosokomial Gram negative rod pneumonia. Am J Med 78:34–41
Rapp RP, Young B, Foster TS, Tibbs PA, O’Neal W (1984) Ceftazidime versus tobramycin/ticarcillin in treating hospital acquired pneumonia and bacteremia. Pharmacotherapy 4:211–215
Fagon JY, Chastre J, Hance AJ, Guiguet M, Trouillet JL, Domart Y, Pierre J, Gilbert J (1988) Detection of nosokomial lung infection in ventilated patients: use of a protected specimen brush and quantitative culture technique in 147 patients. Am Rev Respir Dis 138:110–116
Rello J, Ausina V, Ricart M, Castella J, Prats G (1993) Impact of previous antimicrobial therapy on the etiology and outcome of ventilator associated pneumonia Chest 104:1230–1235
Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, Gibert C (1989) Nosokomial pneumonia in patients receiving continuous mechanical ventilation: prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis 139:877–884
Rello J, Quintana E, Ausina V, Castella J, Luquin M, Net A, Prats G (1991) Incidence, etiology and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest 100:439–444
Rello J, Torres A, Ricart M, Valles J, Gonzalez J, Artigas A, Rodriguez-Roisin R (1994) Ventilator associated pneumonia by Staphylococcus aureus: comparison of methicillin resistant and methicillin sensitive episodes. Am J Respir Crit Care Med 150:1545–1549
Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C (1999) The attributable mortality and morbidity of ventilator associated pneumonia in critically ill. Am J Respir Crit Care Med 159:1249–1256
Mosconi PM, Langer M, Cigada M, Mandelli M (1991) Epidemiology and risk factors of pneumonia in critically ill patients. Eur J Epidemiol 7:320–327
Meduri GU, Beals DH, Maijub AG, Baselski V (1991) Protected bronchoalveolar lavage: a new bronchoscopic technique to retrieve uncontaminated distal airway secretions. Am Rev Respir Dis 143:855–864
Dotson RG, Pingleton SK (1993) The effect of antibiotic therapy on recovery of intracellular bacteria from bronchoalveolar lavage in suspected ventilator associated pneumonia. Chest 103:541–546
Torres A, Martos A, Puig de la Bellacasa J, Ferrer M, el-Ebiary M, Gonzalez J, Gene A, Rodriguez-Roisin R (1993) Specificity of endotracheal aspiration, protected specimen brush, and bronchoalveolar lavage in mechanically ventilated patients. Am Rev Respir Dis 147:952–957
Rello J, Sa-Borges M, Correa H, Leal S-R, Baraibar J (1999) Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med 160:608–613
Ioanas M, Cavalcanti M, Ferrer M, Valencia M, Agusti C, Puig De La Bellacasa J, Torres A (2003) Hospital acquired pneumonia: coverage and treatment adequacy of current guidelines. Eur Respir J 22:876–882
Author information
Authors and Affiliations
Corresponding author
Additional information
This article refers to the editorial available at http://dx.doi.org/10.1007/s00134-2696-z
Rights and permissions
About this article
Cite this article
Giantsou, E., Liratzopoulos, N., Efraimidou, E. et al. Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med 31, 1488–1494 (2005). https://doi.org/10.1007/s00134-005-2697-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2697-y