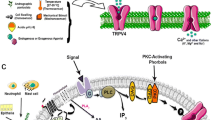
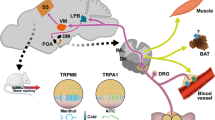
Abstract
Chronic inflammatory diseases are accompanied by a systemic response of the body, necessary to redirect energy-rich fuels to the activated immune system and to induce volume expansion. The systemic response is switched on by two major pathways: (a) circulating cytokines enter the brain, and (b) signals via sensory nerve fibers are transmitted to the brain. Concerning item b, sensory nerve terminals are equipped with a multitude of receptors that sense temperature, inflammation, osmolality, and pain. Thus, they can be important to inform the brain about peripheral inflammation. Central to these sensory modalities are transient receptor potential channels (TRP channels) on sensory nerve endings. For example, TRP vanilloid 1 (TRPV1) can be activated by heat, inflammatory factors (e.g., protons, bradykinin, anandamide), hyperosmolality, pungent irritants, and others. TRP channels are multimodal switches that transmit peripheral signals to the brain, thereby inducing a systemic response. It is demonstrated how and why these TRP channels (TRPV1, TRP ankyrin type 1 (TRPA1), and TRP melastatin type 8 (TRPM8)) are important to start up a systemic response of energy expenditure, energy allocation, and water retention and how this is linked to a continuously activated immune system in chronic inflammatory diseases.






Similar content being viewed by others
References
Straub RH, Cutolo M, Buttgereit F, Pongratz G (2010) Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med 267:543–560
Straub RH (2012) Evolutionary medicine and chronic inflammatory state—known and new concepts in pathophysiology. J Mol Med 90:523–534
Straub RH, Besedovsky HO (2003) Integrated evolutionary, immunological, and neuroendocrine framework for the pathogenesis of chronic disabling inflammatory diseases. FASEB J 17:2176–2183
Pacheco-Lopez G, Bermudez-Rattoni F (2011) Brain-immune interactions and the neural basis of disease-avoidant ingestive behaviour. Philos Trans R Soc Lond B Biol Sci 366:3389–3405
Straub RH (2011) Concepts of evolutionary medicine and energy regulation contribute to the etiology of systemic chronic inflammatory diseases. Brain Behav Immun 25:1–5
Dubner R, Bennett GJ (1983) Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci 6:381–418
Schaible HG, Schmidt RF (1985) Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol 54:1109–1122
Schaible HG, Schmidt RF (1988) Excitation and sensitization of fine articular afferents from cat's knee joint by prostaglandin E2. J Physiol Lond 403:91–104
Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R (1994) Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III 317:499–503
Lanteri-Minet M, Weil-Fugazza J, de Pommery J, Menetrey D (1994) Hindbrain structures involved in pain processing as revealed by the expression of c-Fos and other immediate early gene proteins. Neuroscience 58:287–298
Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF (1995) Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett 183:27–31
Gogas KR, Cho HJ, Botchkina GI, Levine JD, Basbaum AI (1996) Inhibition of noxious stimulus-evoked pain behaviors and neuronal fos-like immunoreactivity in the spinal cord of the rat by supraspinal morphine. Pain 65:9–15
Brenn D, Richter F, Schaible HG (2007) Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum 56:351–359
Boettger MK, Hensellek S, Richter F, Gajda M, Stockigt R, von Banchet GS, Brauer R, Schaible HG (2008) Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum 58:2368–2378
Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB (2009) The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61:228–261
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Schaible HG, Grubb BD (1993) Afferent and spinal mechanisms of joint pain. Pain 55:5–54
Hökfelt T, Kellerth JO, Nilsson G, Pernow B (1975) Substance p: localization in the central nervous system and in some primary sensory neurons. Science 190:889–890
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–135
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Story GM et al (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58
Peier AM et al (2002) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715
Saito S, Shingai R (2006) Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics 27:219–230
Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA (2010) Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464:597–600
Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, Grutzner F, Kaessmann H (2014) Origins and functional evolution of Y chromosomes across mammals. Nature 508:488–493
Kwan KY, Corey DP (2009) Burning cold: involvement of TRPA1 in noxious cold sensation. J Gen Physiol 133:251–256
Daniels RL, McKemy DD (2007) Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Mol Pain 3:23
Steiner AA et al (2007) Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27:7459–7468
Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with a delta/c-fibers and colocalization with trk receptors. J Comp Neurol 493:596–606
Dhaka A, Earley TJ, Watson J, Patapoutian A (2008) Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28:566–575
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P (2007) Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol 7:11
Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 115:2393–2401
Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS (2005) Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain 1:17
Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F (2005) Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci 25:11322–11329
Russell FA, Fernandes ES, Courade JP, Keeble JE, Brain SD (2009) Tumour necrosis factor alpha mediates transient receptor potential vanilloid 1-dependent bilateral thermal hyperalgesia with distinct peripheral roles of interleukin-1beta, protein kinase C and cyclooxygenase-2 signalling. Pain 142:264–274
Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S (2006) Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett 397:140–144
Benedikt J, Teisinger J, Vyklicky L, Vlachova V (2007) Ethanol inhibits cold-menthol receptor TRPM8 by modulating its interaction with membrane phosphatidylinositol 4,5-bisphosphate. J Neurochem 100:211–224
Zakharian E, Cao C, Rohacs T (2010) Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci 30:12526–12534
Bavencoffe A et al (2010) The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway. J Biol Chem 285:9410–9419
Kim J, Cowan A, Lisek R, Raymondi N, Rosenthal A, Hirsch DD, Rawls SM (2011) Icilin-evoked behavioral stimulation is attenuated by alpha-adrenoceptor activation. Brain Res 1384:110–117
Romanovsky AA (2007) Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292:R37–R46
Liu H, Prugnolle F, Manica A, Balloux F (2006) A geographically explicit genetic model of worldwide human-settlement history. Am J Hum Genet 79:230–237
Blaxter K (1989) Energy metabolism in animals and man. Cambridge University Press, Cambridge
Gold AJ, Zornitzer A, Samueloff S (1969) Influence of season and heat on energy expenditure during rest and exercise. J Appl Physiol 27:9–12
Leppaluoto J, Tuominen M, Vaananen A, Karpakka J, Vuori J (1986) Some cardiovascular and metabolic effects of repeated sauna bathing. Acta Physiol Scand 128:77–81
Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T (1996) Endotoxin shock: thermoregulatory mechanisms. Am J Physiol 270:R693–R703
Kobayashi A, Osaka T, Namba Y, Inoue S, Lee TH, Kimura S (1998) Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am J Physiol 275:R92–R98
Ludy MJ, Moore GE, Mattes RD (2012) The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses 37:103–121
Luo Z et al (2012) TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res 22:551–564
Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, Higashi T, Tominaga M, Inoue K, Fushiki T (2009) Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem 73:2690–2697
Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M (2009) Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr 89:45–50
Mori N, Kawabata F, Matsumura S, Hosokawa H, Kobayashi S, Inoue K, Fushiki T (2011) Intragastric administration of allyl isothiocyanate increases carbohydrate oxidation via TRPV1 but not TRPA1 in mice. Am J Physiol Regul Integr Comp Physiol 300:R1494–R1505
Watanabe T, Kawada T, Kurosawa M, Sato A, Iwai K (1988) Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am J Physiol 255:E23–E27
Gavva NR et al (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210
Motter AL, Ahern GP (2008) TRPV1-null mice are protected from diet-induced obesity. FEBS Lett 582:2257–2262
Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA (2011) Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 31:1721–1733
Zhang LL et al (2007) Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res 100:1063–1070
Guillot E, Coste A, Angel I (1996) Involvement of capsaicin-sensitive nerves in the regulation of glucose tolerance in diabetic rats. Life Sci 59:969–977
Moesgaard SG, Brand CL, Sturis J, Ahren B, Wilken M, Fleckner J, Carr RD, Svendsen O, Hansen AJ, Gram DX (2005) Sensory nerve inactivation by resiniferatoxin improves insulin sensitivity in male obese Zucker rats. Am J Physiol Endocrinol Metab 288:E1137–E1145
Kang JH, Goto T, Han IS, Kawada T, Kim YM, Yu R (2010) Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity (Silver Spring) 18:780–787
Koopmans SJ, Leighton B, DeFronzo RA (1998) Neonatal de-afferentation of capsaicin-sensitive sensory nerves increases in vivo insulin sensitivity in conscious adult rats. Diabetologia 41:813–820
Almeida MC, Steiner AA, Branco LG, Romanovsky AA (2006) Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci 23:3359–3367
Ding Z, Gomez T, Werkheiser JL, Cowan A, Rawls SM (2008) Icilin induces a hyperthermia in rats that is dependent on nitric oxide production and NMDA receptor activation. Eur J Pharmacol 578:201–208
Masamoto Y, Kawabata F, Fushiki T (2009) Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci Biotechnol Biochem 73:1021–1027
Tajino K, Matsumura K, Kosada K, Shibakusa T, Inoue K, Fushiki T, Hosokawa H, Kobayashi S (2007) Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am J Physiol Regul Integr Comp Physiol 293:R2128–R2135
Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD (2011) Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 6:e25894
Almeida MC et al (2012) Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32:2086–2099
Ma S et al. (2012) Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol
Sharif-Naeini R, Witty MF, Seguela P, Bourque CW (2006) An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9:93–98
Ciura S, Bourque CW (2006) Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26:9069–9075
Allen DE, Gellai M (1993) Mechanisms for the diuresis of acute cold exposure: role for vasopressin? Am J Physiol 264:R524–R532
Deuster PA, Smith DJ, Smoak BL, Montgomery LC, Singh A, Doubt TJ (1989) Prolonged whole-body cold water immersion: fluid and ion shifts. J Appl Physiol 66:34–41
Fregly MJ (1982) Water and electrolyte exchange during exposure to cold. Pharmacol Ther 18:199–231
Sun Z (2006) Genetic AVP deficiency abolishes cold-induced diuresis but does not attenuate cold-induced hypertension. Am J Physiol Ren Physiol 290:F1472–F1477
Ferguson AV, Pittman QJ, Riphagen CL (1984) Effect of cooling on supraoptic neurohypophysial neuronal activity and on urine flow in the rat. J Physiol 352:103–112
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702
Gevaert T et al (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117:3453–3462
Tsushima H, Mori M (2006) Antidipsogenic effects of a TRPV4 agonist, 4alpha-phorbol 12,13-didecanoate, injected into the cerebroventricle. Am J Physiol Regul Integr Comp Physiol 290:R1736–R1741
Kukkonen-Harjula K, Kauppinen K (1988) How the sauna affects the endocrine system. Ann Clin Res 20:262–266
Lammintausta R, Syvalahti E, Pekkarinen A (1976) Change in hormones reflecting sympathetic activity in the Finnish sauna. Ann Clin Res 8:266–271
Tatar P, Vigas M, Jurcovicova J, Jezova D, Strec V, Palat M (1985) Impaired glucose utilization in man during acute exposure to environmental heat. Endocrinol Exp 19:277–281
Pilch W, Szygula Z, Klimek AT, Palka T, Cison T, Pilch P, Torii M (2010) Changes in the lipid profile of blood serum in women taking sauna baths of various duration. Int J Occup Med Environ Health 23:167–174
Kauppinen K (1989) Sauna, shower, and ice water immersion. Physiological responses to brief exposures to heat, cool, and cold. Part I. Body fluid balance. Arctic Med Res 48:55–63
Pereira CT, Herndon DN (2005) The pharmacologic modulation of the hypermetabolic response to burns. Adv Surg 39:245–261
Holland-Fischer P, Greisen J, Grofte T, Jensen TS, Hansen PO, Vilstrup H (2009) Increased energy expenditure and glucose oxidation during acute nontraumatic skin pain in humans. Eur J Anaesthesiol 26:311–317
Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N, Szekely M (1997) Cold defense mechanisms in vagotomized rats. Am J Physiol 273:R784–R789
Knitza R, Olbermann M, Fischer F, Bassler KH (1978) Changes of circulation, blood gases, acid-base-and metabolism during neurolept-analgesia with and without beta-receptor blockers in electro coagulation of Gasser's ganglion (author's transl). Anaesthesist 27:213–218
Knitza R, Clasen R, Fischer F (1979) Pain-induced alterations in the individual non-esterified fatty acids in serum. Pain 6:91–97
Carlstrom S, Christensson B (1971) Plasma glycerol concentration in patients with myocardial ischemia and arrhythmias. Br Heart J 33:884–888
Greisen J, Juhl CB, Grofte T, Vilstrup H, Jensen TS, Schmitz O (2001) Acute pain induces insulin resistance in humans. Anesthesiology 95:578–584
Kelsall AR (1949) The inhibition of water diuresis in man by ischaemic muscle pain. J Physiol 109:150–161
Sakemi T, Ikeda Y, Matsuo Y, Kudo S, Nishihara G, Baba N (1996) Renal wedge-shaped lesions on computed tomography and ultrasonography in two patients who developed acute renal failure with severe loin pain after exercise. Nephron 73:679–681
Barber A et al (1994) A pharmacological profile of the novel, peripherally-selective kappa-opioid receptor agonist, EMD 61753. Br J Pharmacol 113:1317–1327
Friedrich M, Meyberg R, Friedrich G, Axt R, Villena-Heinsen C (2000) Evaluation of the secretion of the atrial natriuretic factor (ANF) after laparotomy. Clin Exp Obstet Gynecol 27:113–115
Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM (2011) Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol 186:6417–6426
Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM (2011) LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 90:759–764
Wu ZZ, Pan HL (2007) Role of TRPV1 and intracellular Ca2+ in excitation of cardiac sensory neurons by bradykinin. Am J Physiol Regul Integr Comp Physiol 293:R276–R283
Langeslag M, Constantin CE, Andratsch M, Quarta S, Mair N, Kress M (2011) Oncostatin M induces heat hypersensitivity by gp130-dependent sensitization of TRPV1 in sensory neurons. Mol Pain 7:102
Xing J, Lu J, Li J (2009) Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296:H1380–H1387
Ciobanu C, Reid G, Babes A (2009) Acute and chronic effects of neurotrophic factors BDNF and GDNF on responses mediated by thermo-sensitive TRP channels in cultured rat dorsal root ganglion neurons. Brain Res 1284:54–67
Amadesi S et al (2004) Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24:4300–4312
Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ (2005) A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A 102:4536–4541
Basu S, Srivastava P (2005) Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A 102:5120–5125
Andersson DA, Gentry C, Moss S, Bevan S (2008) Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28:2485–2494
Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118:1899–1910
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Keeble J, Russell F, Curtis B, Starr A, Pinter E, Brain SD (2005) Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum 52:3248–3256
Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M (2005) Attenuated fever response in mice lacking TRPV1. Neurosci Lett 378:28–33
Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G (2007) The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci 36:381–391
Chung MK, Lee J, Duraes G, Ro JY (2011) Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res 90:1103–1107
Segond von Banchet G, Richter J, Huckel M, Rose C, Brauer R, Schaible HG (2007) Fibroblast-like synovial cells from normal and inflamed knee joints differently affect the expression of pain-related receptors in sensory neurones: a co-culture study. Arthritis Res Ther 9:R6
Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schafer M, Stein C, Zollner C (2007) Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol 71:12–18
Chen Y, Willcockson HH, Valtschanoff JG (2009) Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthr Cartil 17:244–251
Kimball ES, Wallace NH, Schneider CR, D'Andrea MR, Hornby PJ (2004) Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil 16:811–818
Szitter I, Pozsgai G, Sandor K, Elekes K, Kemeny A, Perkecz A, Szolcsanyi J, Helyes Z, Pinter E (2010) The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J Mol Neurosci 42:80–88
Lee LY, Gu Q (2009) Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 9:243–249
Liddle RA (2007) The role of transient receptor potential vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta 1772:869–878
Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ (2007) Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 133:1282–1292
Razavi R et al (2006) TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127:1123–1135
Okada Y, Reinach PS, Shirai K, Kitano A, Kao WW, Flanders KC, Miyajima M, Liu H, Zhang J, Saika S (2011) TRPV1 involvement in inflammatory tissue fibrosis in mice. Am J Pathol 178:2654–2664
Engel MA et al (2011) TRPA1 and substance P mediate colitis in mice. Gastroenterology 141:1346–1358
Fernandes ES et al (2011) A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund's complete adjuvant-induced monarthritis. Arthritis Rheum 63:819–829
da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB (2010) The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 148:431–437
Caceres AI et al (2009) A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A 106:9099–9104
Ramachandran R et al (2013) TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A 110:7476–7481
Acknowledgments
I want to express my sincere appreciation to Prof. Dr. David Pisetsky, Duke University Medical Center, North Carolina, who provided helpful editorial comments. The author has been supported over many years by grants from the DFG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Straub, R.H. TRPV1, TRPA1, and TRPM8 channels in inflammation, energy redirection, and water retention: role in chronic inflammatory diseases with an evolutionary perspective. J Mol Med 92, 925–937 (2014). https://doi.org/10.1007/s00109-014-1175-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1175-9