Summary
This review deals with the avian paramyxovirus Newcastle disease virus (NDV) and describes properties that explain its oncolytic activity, its tumor-selective replication behavior, and its immune-stimulatory capacity with human cells. The strong interferon response of normal cells upon contact with NDV appears to be the basis for the good tolerability of the virus in cancer patients and for its immune stimulatory properties, whereas the weak interferon response of tumor cells explains the tumor selectivity of replication and oncolysis. Various concepts for the use of this virus for cancer treatment are pointed out and results from clinical studies are summarized. Reverse genetics technology has made it possible recently to clone the genome and to introduce new foreign genes thus generating new recombinant viruses. These can, in the future, be used to transfer new therapeutic genes into tumors and also to immunize against new emerging pathogens. The modular nature of gene transcription, the undetectable rate of recombination, and the lack of a DNA phase in the replication cycle make NDV a suitable candidate for the rational design of a safe and stable vaccine and gene therapy vector.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key Words
1. Introduction
Newcastle disease virus (NDV) is one of five species of viruses that are under clinical evaluation as vectors for oncolytic cancer therapy, for gene therapy, and for immune stimulation (1–3). The concept of using viruses to treat human cancer dates back to the early 1900s. The rationale receives support from well-documented remissions of malignancies induced by natural viral infections (measles and mumps or upon vaccination with attenuated viral vaccines) (4–7). These observations stimulated a transient peak of research activity in the 1960s and 1970s and received a new interest in the 1980s and 1990s on the basis on new preclinical and clinical (7–14) results. Among all the viruses used, RNA viruses are rapidly emerging as particularly promising agents for virus therapy of cancer (15). Integral to the live cycle of all RNA viruses is the formation of double-stranded RNA (dsRNA), which activates a spectrum of cellular defence mechanisms involving type I interferons: interferon (IFN)-α and IFN-β. Tumors provide a relatively permissive substrate for the propagation of RNA viruses because mutations in tumor cells often cripple the interferon system to allow uninhibited proliferation and to provide resistance to apoptosis (16). The most promising RNA viruses are attenuated strains of mumps virus, NDV, measles virus, vesicular stomatitis virus, human reovirus, poliovirus, and influenza virus (17). Although human RNA viruses had the ability during evolution to adapt to the human immune system and to develop immune escape mechanisms, the advantage of NDV is that it is a bird virus that has adapted only to the avian immune system (18, 19).
The virus name comes from the site of the first reported disease outbreak among chickens, at a farm near Newcastle-upon-Tyne in England in 1926 (20, 21). NDV is now classified as an avian paramyxovirus (APMV)-1 in the Rubulavirus genus of the family Paramyxoviridae in the order Mononegaviralis (22). NDV is an enveloped virus of 100- to 300-nm diameter with a negative-sense single-stranded RNA genome of roughly 16,000 nucleotides. These code for six genes encoding six major polypeptides: the large protein (L, 200 kDa), the hemagglutinin–neuraminidase protein (HN, 74 kDa); the fusion protein (F, 67 kDa); the matrix protein (M, 40 kDa); the phosphoprotein (P, 53 kDa); and the nucleoprotein (NP, 55 kDa). By means of an overlapping reading frame, the P gene encodes for an additional gene product, the V protein. The RNA-dependent RNA polymerase involves the proteins L, P, and NP, which are translated in infected cells at free ribosomes in the cytoplasm. The F glycoprotein is synthesized as an inactive precursor (F0, 67 kDa), which undergoes proteolytic cleavage to yield the biologically active protein consisting of the disulfide-linked chains F1 (55 kDa) and F2 (12.5 kDa) (for more details about the classification and the structure of NDV, see ref. 23).
The first hints of the potential anticancer benefit of this virus in patients were made more than 40 years ago. In 1964, Wheelock and Dingle reported a significant reduction in leukemic blasts in a patient with myelogenous leukemia treated by intravenous (i.v.) administration of NDV (24). In 1965, Cassel and Garrett reported the effects of intratumoral NDV treatment of a patient with cervical cancer (25). Marked tumor shrinkage of the injected mass as well as the supraclavicular lymph node metastasis was observed and the patient tolerated the treatment well. In 1971, Csatary noted that a chicken farmer, shortly after a known exposure to NDV from his flock of infected chickens, had a spontaneous remission of his metastatic cancer (26). In the same report, he noted tumor regression in three other patients, all of whom were intentionally inoculated with NDV. Since this time, a certain number of clinical studies using this virus for cancer therapy have been performed (27). NDV has been labeled as a complementary and alternative medicine (CAM) by the National Cancer Institute (NCI) in the USA. More detailed information can be found at the home page of the NCI (28).
Since the first observations showing antineoplastic properties of NDV, different concepts have been investigated or are in the process of being developed regarding the use of NDV for cancer treatment or for vaccination purposes (24, 25):
-
1.
Use for tumor selective cytolysis (viral oncolysis) (29)
-
2.
Use for nonspecific immune stimulation (induction of cytokines and interferons) (4)
-
3.
Use as adjuvant and danger signal (DS) in a tumor vaccine for stimulation of cytotoxic T lymphocyte (CTL) and delayed-type hypersensitivity (DTH) responses (30)
-
4.
Use as viral vector for the transfer of therapeutic genes (31)
-
5.
Use as vaccine vector for immunization against emerging pathogens (32)
2. Mechanism of Action
2.1 Infection and Replication of NDV
Infection of cells by NDV can be schematically divided into two steps, which are illustrated in Fig. 1 . The first step involves the binding of the virus to a host cell’s surface via the cell-binding domain of the HN molecule. This is followed by the activation of the fusion protein F. The concerted action of HN and F leads to fusion of the viral and the host cell membrane. This membrane fusion event allows the viral genome to enter the cytoplasm of the host cell. There, the negative-stranded RNA genome is transcribed into messenger RNA (mRNA) and translated to viral proteins (first step). The proteins NP, P, and L are required for nucleocapsid assembly. The nucleocapsid as “antigenome” is then used as a template for viral replication (second step). The M protein and the envelope proteins HN and F, after posttranslational modification, move to the membrane, where virus assembly and budding occurs (33, 34). In this process, single copies of the NDV genome become wrapped into an outer coat envelope that is made from host cells’ plasma membrane. Efficient replication has been observed to be dependent on the genome length being a multiple of six. This requirement is known as the “rule of six” (35). It is assumed that each NP subunit is in contact with exactly six nucleotides and that this arrangement is required for efficient replication (36).
Cell infection by NDV. NDV replication is composed of two steps (left). The first step consists, after binding and fusion of the virus with the target cell, in the transcription of the viral genes (coding for the nucleoprotein [NP], the phosphoprotein [P], the matrix protein [M], the hemagglutinin–neuraminidase protein [HN], and the large [L] proteins) and in their transaction (right). The second step corresponds to the amplification of the viral genome. The antigenome RNA is used as template for the synthesis of the new viral genomes. The viral RNA-dependent RNA polymerase is composed of the association of the two viral proteins P and L. Encapsulation of the viral genomes occurs at the plasma membrane, from which new virus particles are released via budding from the infected cells (for more details, see the main text, (33), and (167)).
This representation may be too simplistic because it has been shown recently that enveloped viruses such as NDV can enter the cell through two main pathways:
-
(i))
Via direct fusion between the envelope and the plasma membrane as described above (23). For NDV, it has been established that the membrane fusion process takes place at the host plasma membrane in a pH-independent manner (37). Activation of the fusion protein F occurs through interaction of the viral glycoproteins with the sialic acid-containing cellular receptors such as gangliosides and N-glycoproteins, which are ubiquitously expressed on cell surfaces (38–40).
-
(ii))
Via receptor-mediated endocytosis (41). It was recently shown that NDV might also infect cells through a caveolae-dependent endocytic pathway as an alternative route (41). A certain percentage of virions might be endocytosed to endosomes, where fusion would occur at lowered pH (41).
The ordered assembly and release of infectious NDV particles has been shown to depend on membrane lipid rafts (42). These are defined as cholesterol- and sphingolipid-rich microdomains in the exoplasmic leaflet of cellular plasma membranes (42). Newly produced virions showed an accumulation of HN, F, and NP viral proteins in lipid rafts early after synthesis and contained the lipid raft-associated proteins caveolin-1, flotillin-2, and actin, but not the non-lipid raft-associated transferring receptor (42). The assembly and release of NDV virus-like particles (VLPs) from avian cells expressing all possible combinations of viral proteins revealed that M protein is necessary and sufficient for VLPs (43). M-HN and M-NP interactions were responsible for incorporation into VLPs, and F proteins were incorporated due to interactions with NP and HN proteins (43).
2.2 Tumor Selectivity of NDV
Although, in nontumorigenic human T cells (even activated in culture with interleukin [IL]-2), the replication cycle of NDV is stopped, tumor cells continue in the replication cycle and produce viral particles within 10–50 h after infection ((30); see Fig. 2 ). Certain NDV strains can replicate up to 10,000 times better in human neoplastically transformed cells than in most normal human cells (30). This appears to be the basis of the widely accepted fact that the virus is virtually nontoxic in human and for the interest in its use as a form of cancer therapy. Most remarkable is the strong capacity of NDV to induce type I interferon responses by viral protein and RNA (44) (Fig. 3 ). Viral RNA replication induces in infected cells an innate antiviral program that initiates the transcription of RNA-responsive genes. The responses involve a multimodal machinery of gene regulation by the interferon regulatory factor (IRF) family of transcription factors (45). NDV stimulates, in human peripheral blood mononuclear cells (PBMC), interferon-induced genes (ISG) such as the antiviral enzymes protein kinase R (PKR), a dsRNA-responsive protein kinase; MxA, a dynamin-like GTPase with antiviral activity; and RNaseL (46–48). The latter was recently shown to generate small self-RNA, thereby further amplifying antiviral innate immunity (49).
Observed differences between interaction of NDV with normal and tumor cells. NDV shows a different pattern of replication in normal cells (left) when compared with tumor cells (right). Its weak replication in normal cells can be correlated with an efficient antiviral response within the infected cells. In contrast, its efficient replication in tumor cells is linked to a weak antiviral response of the latter. These data are based on the analysis of the evolution of the level of genomic (negative) and antigenomic (positive) RNA within the cells during infection (47). One hour after infection, no difference can be observed between normal and tumor cells (47). Twelve hours after infection, the amount of genomic viral RNA was lower in normal cells than in tumor cells (47). The interferon-induced genes (ISG) were also induced differently in normal and tumor cells (for more details, please refer to (47)).
Monocyclic versus multicyclic replication patterns of NDV in tumor cells. Left: Non-lytic NDV (for example, the strain Ulster), when added onto a monolayer of tumor cells, leads to the production of viral particles that cannot infect other tumor cells: abortive monocyclic replication. Right: In contrast, lytic NDV strains (for example, the strain MTH-68) lead, after infection of tumor cells, to the production of infectious particles that can infect other tumor cells, thereby leading to an amplification of the viral load: a multicyclic replication cycle.
To clarify the underlying mechanism of the observed difference in virus susceptibility to infection of tumor cells and nontumor cells, we examined the kinetics of interferon-induced antiviral enzymes. The analysis revealed several defects of tumor cells in their antiviral defence responses: they showed no response to UV-inactivated NDV, whereas nontumorigenic cells reacted with induction of high levels of the antiviral enzymes PKR and MxA (47). We conclude that the induction of an early and efficient antiviral response in nontumorigenic cells could explain the stop of the replication cycle of NDV after the production of positive-strand RNA (47). In contrast, the tumor cells show a weak and delayed antiviral response (47, see Fig. 2 ). This observation may explain the continuation of the replication cycle, which leads to high expression of the viral proteins (47).
2.3 Oncolytic Properties of NDV
NDV strains suitable for antineoplastic therapy can be classified as either lytic or non-lytic for human cells (Fig. 3 ). Both lytic strains and non-lytic strains replicate much more efficiently in human neoplastic cells than they do in most normal human cells. Viruses of both strain types have been investigated as potential anticancer agents. One major difference between lytic and non-lytic strains is that lytic strains are able to make infectious progeny virus particles in human neoplastic cells, whereas non-lytic strains are not (50). The reason for that is that the progeny virus particles made by non-lytic strains contain inactive versions of F molecules. Advantages of the lytic properties of some NDV strains is that the production of infectious progeny virus particles, after a first round of viral infection, gives them the ability to spread the virus in tumor tissues via multicyclic replication (see Fig. 3 , right). In contrast, non-lytic strains can only perform a monocyclic replication cycle (see Fig. 3 , left).
Oncolytic NDV strains are cytotoxic to human tumor cell lines of ectodermal, endodermal, and mesodermal origin (51). They exert oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death (52). NDV infection has been shown to result in the loss of mitochondrial membrane potential and release of mitochondrial protein cytochrome C (51). In addition, it leads to early activation of caspase 9 and caspase 3. In contrast, cleavage of caspase 8, which is predominantly activated by the death receptor pathway, is a late event in NDV-mediated apoptosis of tumor cells that is induced by tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (51). The death signals generated by NDV in tumor cells ultimately converge at the mitochondria (51).
A recent study revealed that a human glioblastoma cell line with repressible expression of the p53 protein did not show any difference in NDV sensitivity in its p53-expressing and p53-depleted cells (52). This indicates that the apoptotic process induced by NDV does not depend on p53. In two human tumor cell lines, virus replication led to signs of endoplasmic reticulum stress such as phosphorylation of protein kinase R (PKR)-like endoplasmic reticulum kinase and elongation factor 2 alpha (eIF2α) (52). Thus, in vitro, oncolytic NDV selectively kills tumor cell cultures by inducing endoplasmic reticulum stress leading to p53-independent apoptotic cell death (52).
Such cytotoxic properties have raised considerable interest in recent years for clinical application of such oncolytic NDV viruses (1, 19, 29, 53). In vivo tumoricidal activity was evaluated in athymic nude mice by subcutaneous injection of 9 × 106 tumor cells followed by intralesional injection of 1 × 106 plaque forming units (pfu) of oncolytic NDV (8). This treatment caused complete tumor regression in athymic mice with a high therapeutic index (54). Recently, we characterized virological, immunological, and antitumor properties of some NDV strains. We observed that the oncolytic strain MTH-68/H was the most potent IFN-α inducer among all NDV strains tested (54). After ultraviolet light (UV) inactivation, this strain was the only one tested that could induce in human PBMC antitumor activity in vitro (54). Upon systemic application of high doses of NDV to mice bearing a virus-susceptible intradermal tumor, no significant antitumor effects were observed with the two oncolytic strains Italien and MTH68/H (54). The treatment had nevertheless significant side effects as seen by loss of body weight (54). In contrast, when using a locoregional application system for treatment of liver metastases of luciferase-transfected murine CT26 colon carcinoma cells, MTH68/H showed a significant delay in tumor growth as well as prolonged survival but no effects on body weight (54). Surprisingly, this murine tumor cell transfectant was resistant in vitro to virus infection and oncolysis (54). These results suggest:
-
(i)
That locoregional application of oncolytic NDV is more effective than systemic i.v. application and
-
(ii)
That oncolytic NDV may mediate effects against a virus-resistant tumor line, possibly via host-mediated mechanisms
For explanation for the in vivo mechanisms induced by oncolytic NDV strains, we therefore have to include host-mediated mechanisms. Those will be dealt within the following section in detail.
2.4 Properties of Immune Modulation of NDV
The immunostimulatory properties of NDV have long been recognized, although the exact mechanism leading to the activation of the human immune system is still under investigation (55, 56).
2.4.1 Activation of Innate Immunity
NDV and Natural Killer (NK) cells
Human NK cells can be activated to antitumor cytotoxic activity by NDV infected tumor cells in contrast to uninfected tumor cells. Such activated NK cells exert in vitro significant bystander antitumor activity when stimulation cultures are performed on top of human tumor cell monolayers (57). The antiviral response by NK cells involves induction of TRAIL by IFN-α/β (58).
NDV and Macrophages/Monocytes
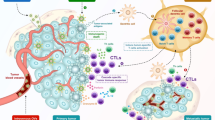
NDV has been shown also to activate murine macrophages so that various macrophage enzymes (adenosine deaminase [ADA], inducible nitric oxide synthase [iNOS], lysozyme, acid phosphatase) become upregulated and antitumor effector molecules such as nitric oxide (NO) and tumor necrosis factor (TNF)-α are detected in the supernatant (59). NDV-activated macrophages performed antitumor cytotoxicity in vitro against various tumor lines including immune escape variants (59). In addition, repeated intravenous transfer of NDV-activated macrophages were observed to exert a significant suppressive effect on pulmonary metastases of mammary and lung carcinomas (59)(Fig. 4 ).
Interactions of NDV with cells of the innate human immune system. NDV, when present at the surface of infected tumor cells, interacts with cells of the innate immunity. It induces the expression of TRAIL at the surface of NK cells and monocytes. It leads also to the production of nitric oxide (NO) and tumor necrosis factor-α (TNF-α) by macrophages. This enhances antitumor immunity (for more details, see the main text).
Induction of NO synthesis in macrophages by NDV is associated with activation of nuclear factor-kB (NF-kB) (60). These reactions are part of an activation process that includes stimulation of ADA and inhibition of 5` nucleotidase. They suggest that signaling requirements of NF-kB activation and NO production are similar in NDV-activated macrophages (60).
In another study, TRAIL was shown to mediate tumoricidal activity of human monocytes stimulated by NDV (61). Within 4 h of co-incubation of monocytes with NDV, a strong induction of mRNA for TRAIL is observed (61). After 14 h, NDV-activated monocytes exerted antitumor cytotoxic activity and killed TRAIL-R2 receptor-expressing tumor lines (61). This cytotoxic activity could be partially blocked by soluble TRAIL-Fc but not by recombinant TNF-α–Fc fusion proteins or inhibitors of iNOS (61).
2.4.2 Activation of Adaptive Immunity by NDV
NDV and Dendritic Cells (DCs)
The task of DCs consists of the uptake, processing, and presentation of antigens as well as the recognition of danger signals. DCs can be produced in great numbers from PBMC-derived monocytes (MoDCs) and the loading of DCs with antigen can be done in vivo as well as in vitro. dsRNA motifs have been described to lead to maturation, activation, and protection of DCs (62). Antigens from tumor cells can be associated with danger signals from virus infection by the use of virus-infected tumor cells or oncolysates for DC loading (Fig. 5 ).
Interactions of NDV with cells of the adaptive human immune system. NDV induces the activation of dendritic cells (DC) that may result in the enhancement of uptake and presentation of tumor-associated peptides loaded on major histocompatibility complex (MHC) molecules to T cells (63). The viral HN protein, via its interaction with T cells, induces costimulatory stimuli (69), thereby activating already existing memory T cells specific for the tumor cells (63). We observed also an increase of TRAIL expression on T cells (100) and a Th1 polarization of the antitumoral response (for more details, see the main text and the references 59, 60, and 61).
Dendritic cells pulsed with viral oncolysates were reported to potently stimulate autologous T cells from cancer patients (63). In this study, DCs from breast cancer patients were pulsed with lysates from the MCF-7 breast cancer line (Tu-L) or from NDV-infected MCF-7 cells (TuN-L, viral oncolysate) and compared for stimulatory capacity in an enzyme-linked immuno spot technique (ELISPOT) response of autologous bone marrow (BM)-derived memory T cells (63). DCs pulsed with viral oncolysates showed increased expression of co-stimulatory molecules (as CD86, CD40, and CD40L) and induced significantly higher ELISPOT memory T cell responses (63). Supernatants from such cocultures contained increased titers of IFN-γ, IFN-α, and interleukin (IL)-15 in comparison with cocultures of T cells and DCs pulsed with noninfected tumor lysate (63). IL-15 is known to drive monocyte differentiation toward DCs and also to have an important effect on cell proliferation and maintenance of memory CD8 T cells (64). It has recently been described that IL-15-induced human DCs efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL (65).
These results suggest that a DC preparation pulsed with viral oncolysate includes danger signals (e.g., dsRNA, cytokines, heat-shock proteins) and is superior for T cell stimulation to a DC preparation pulsed with lysate from noninfected tumor cells (63).
NDV and T Lymphocytes
NDV has also an effect on T lymphocytes. Infection of murine ESb tumor cells with low amounts of NDV was sufficient to lead to an increase in cytolytic activity of tumor-specific CTL after sensitization in vivo and restimulation in vitro (66). The number of ESb-specific CTL per spleen could be raised from 3,300 to 9,100 as tested by limiting dilution analysis. In split-type experiments, it could be shown, at the clonal level, that viral modification did not alter the specificity of ESb-specific CTL (66). Thus, NDV modification of tumor cells led to a selective augmentation of TAA-specific CTL (66).
In another study, modification of tumor cells by a low dose of NDV led to augmented tumor-specific T cell response as a result of CD4 and CD8 immune T cell cooperation (67). ESb-NDV-immune CD4 helper T cells produced more IL-2 after antigen stimulation than the corresponding ESb immune cells (67). The virus-mediated augmentation of the CD8 CTL response involved increased T helper activity but did not involve the recognition of viral epitopes as potential new helper determinants. The term “viral xenogenization” of tumor cells is thus inappropriate to describe the mechanism of function of NDV in the tumor vaccine (67).
Postoperative activation of tumor-specific CTL precursors (CTLp) from mice with metastases required stimulation with the specific antigen to which additional signals have been incorporated (67). The analysis of the immune status of spleens from tumor-bearing or tumor-immune mice revealed important differences (67). The activation of CTLp from mice with metastases but not from tumor-immune mice required helper factors (such as IL-2 or NDV (68)).
We studied also the underlying mechanism of T cell stimulation in more detail. The viral HN protein of NDV was shown to augment the peptide-specific cytotoxic T cell response (69). A greater than sixfold increase in influenza nucleoprotein peptide (corresponding to the amino acids 50–63 of this protein)-specific CTL responses was observed in cultures restimulated with peptide-pulsed syngeneic Ltk fibroblast cells that coexpressed the viral HN protein due to either infection or to HN complementary DNA (cDNA) transfection (69). These findings suggest that HN, when expressed on antigen-presenting cells or tumor cells, can exert a T cell co-stimulatory function (69).
NDV infection of human melanoma cells was reported to mediate T cell co-stimulatory function and to break anergy (70). Viral oncolysates of NDV have been widely used for the treatment of malignant melanoma. Melanoma cells and tumor-infiltrating CD4 T lymphocytes (TILs) were prepared from freshly resected tumors, and TILs were subjected to limiting dilution cloning (70). One T helper clone was unresponsive to stimulation with autologous tumor cells and remained unresponsive even to subsequent stimulation by IL-2 (70). NDV infection of the melanoma cells not only completely restored the proliferative response of the helper T cells but also prevented the induction of anergy (70). Electrophoretic mobility shift assays revealed the induction of the CD28-responsive complex in the T cells by co-incubation with NDV-infected melanoma cells (70). Furthermore, the activation of the CD28 pathway did not involve B7-1/B7-2 ligands (70).
In other assays, tumor cells infected by NDV showed an increased adhesiveness for erythrocytes and lymphocytes (71). The increased adhesion could be blocked by a monoclonal antibody (mAb) against HN but not against F (71). HN cDNA transfectants also mediated increased lymphocyte adhesion (71). A mutant of the NDV strain Australian Victoria (AV-L1) having highly decreased neuraminidase activity was similar to the NDV strain Ulster in adhesive and T cell co-stimulatory function whereas the parental AV strain with high neuraminidase activity of its HN molecule was negative for both functions (71). Co-stimulatory effects of NDV Ulster virions were revealed when virions and suboptimal concentrations of anti-CD3 mAbs were coated to microtiter plates for induction of murine CD4 T cell proliferation (71). In human autologous mixed lymphocytes tumor cultures (MLTC), upregulation of T cell activation markers CD69 and CD25 was observed with NDV modified but not with nonmodified tumor cells (71).
Another explanation for the enhancement of the antitumor immune response by the addition of addition of NDV onto tumor cells was attributed to the fact that human tumor cell infection by NDV was found to lead to upregulation of HLA molecules (72). Infection with live but not inactive NDV induced, in all tumor cells tested, the chemokines RANTES and interferon-γ-inducible protein 10 (IP-10) (72). These chemokines increase chemotaxis and lead to the recruitment of monocytes and T cells to the site of vaccine application. Seventy-two hours after infection of the tumor cells with live NDV Ulster, many tumor cells were dead or in early or late stages of apoptosis (72). Apoptosis is often characterized as a noninflammatory cell death. In the setting of virus infection, apoptosis might, however, promote inflammatory responses and lead to antigen cross priming.
2.4.3 Activation of Type I Interferon Response by NDV
Infections by NDV of cells from the host immune system induce a strong innate immune response characterized by rapid production of type I interferon (IFN-α/β), leading to the inhibition of virus replication. This antiviral response is initiated through the recognition of viral products such as dsRNA, by two types of pathogen recognition receptors: the Toll-like receptors (TLRs) (73) and the RIG-I-like receptors (RLRs) (74). The TLR family consists of more than ten members expressed on the cell surface membrane or on endosomes (73). The RLRs is a family of cytoplasmic RNA helicases that includes RIG-I and MDA-5 (74).
The dsRNA molecules, which are synthesized during NDV replication in the cytoplasm, are recognized by TLR-3 and RIG-1/MDA-5 in a cell type- and pathogen type-specific manner (75, 76). Studies of RIG-1- and MDA-5-deficient mice have revealed that conventional dendritic cells (cDCs), macrophages, and fibroblasts isolated from these mice have impaired type I IFN induction after infection by RNA viruses whereas production of IFN is still observed in plasmacytoid DCs (pDCs) of these mice (76). DCs have been shown to be a cell population able to produce high amounts of interferon-α (77). Thus, the TLR system appears to be required for pDCs to induce the antiviral response whereas for cDCs, macrophages, and fibroblasts, RLRs are critical to sense NDV.
Recently, it was shown that dsRNA in the apoptotic bodies of virus-infected dead cells is recognized by CD8α dendritic cells (DCs) that have high expression of TLR-3 (78). This promotes cross-priming of T cells to virus-infected cells (79). The release of high amounts of interferon-α by PBMC is caused by activation of DCs (both myeloid and plasmacytoid) and of monocytes (31).
It has only recently become clear that IFN-α has an important adjuvant function in the immune response as it was suggested early on (80). An adjuvant effect of type I IFN could explain why most live viruses elicit strong immune responses whereas viral peptides are poorly immunogenic or tolerogenic unless supplemented with exogenous adjuvant (81). IFN-α has been shown to induce (1) TRAIL in NK cells (82) and monocytes and (2) cell-mediated cytotoxicity (61). Interestingly, there is a crosstalk between the IFN signaling pathway and TRAIL (83). This can explain the fact that, in monocytes, NDV upregulates the expression of TRAIL, which mediates their tumoricidal activity (84).
NDV induces danger signals for DCs and stimulates them (63, 85). It consequently improves their antigen-presenting immune stimulatory function. The first published danger model of immunity (86, 87) proposed only one mechanism for immune recognition of danger that is perceived by DCs upon release of cellular contents after necrosis of a diseased cell in its neighborhood. A recently formulated medical hypothesis suggests that also T lymphocytes themselves correlate danger signals to antigen (88). This model associates danger also with non-lytic viruses if these are upregulating danger signals in their host cell (88).
Thus, NDV has several features that enhance its potency as vaccine vector because it induces immunological danger signals at the site of infection. In contrast, other viruses that have adapted to the mammalian immune system have developed virally encoded inhibitors of immunity, such as TAP inhibitors, cytokine decoys, microRNAs, and viral proteins that antagonize type I interferon induction (89, 90).
NDV induces a strong interferon response that involves an early and a late phase. In the early phase, the retinoic acid-inducible gene (RIG-I) (91) has been shown to function in cDCs and tumor cells as a cytoplasmic viral RNA receptor. RIG-I participates in the recognition of paramyxoviruses and orthomyxoviruses (76), whereas MDA-5 is central for recognition of picornaviruses (92). RIG-I binds specifically to RNA containing 5`-phosphate such as viral RNA (93), whereas mammalian mRNA is either capped or contains base modifications. RIG-1 may thus be able to discriminate between self and nonself (viral) RNA (94). RNA-activated RIG-1 binds to the CARD containing adaptor protein IPS-1, which, after a further signal cascade, activates, in the early phase, interferon regulatory factor (IRF)-3, which is then phosphorylated, translocates to the nucleus, and induces interferon responses. In the late phase of the interferon response, the secreted interferon molecules interact with cell surface-expressed type I IFN receptors and initiate an amplification loop of the interferon response that involves STAT proteins and IRF-7 (for reviews, see (95) and (96)).
IFN-α/ß was also found to play an important role in the generation of CTL activity (97). The generation of CTL activity in MLTC assays using ESb-NDV as stimulator cells could be blocked specifically by antisera to type I IFN (97). Similar effects were observed in vivo during CTL priming, suggesting that IFN-α/ß not only increases CTL activity but is essential for the generation of CTL activity (97).
IFN has been reported to induce the IL-12 receptor-β chain in T cells (98). Together with IL-12, IFN-α polarizes the T cell toward a cell-mediated T helper 1 (Th1) response characterized by delayed-type hypersensitivity (DTH) and cytotoxic T lymphocyte (CTL) activity. In addition, IFN-α induces the upregulation of molecules that are important for antigen recognition (e.g., HLA) and cell–cell interaction (e.g., cell adhesion molecules (72)).
Transfection of HN but not F cDNA of NDV to stimulator BHK cells was found to lead to induction of IFN-α in human blood mononuclear cells (99). NDV is an excellent inducer in PBMC of IFN-α and thereby upregulates plasma membrane expression of TRAIL on CD14 monocytes and CD3 T cells. Upon contact with BHK cells expressing HN but not F at their cell surface after high-efficiency transfection, human PBMC were stimulated in a paracrine way to produce IFN-α (99).
The stimulation of human natural IFN-α response of PBMC by NDV was also reported to be based on paramyxovirus hemagglutinin lectin cell interaction. A lectin–carbohydrate recognition event without enzymatic function is likely the molecular basis for an important innate immune response to an enveloped virus. This conclusion is based on two types of experimental evidence: (1) strong UV irradiation of NDV, which destroyed the cell binding and hemadsorption (HAd) but not the neuraminidase (NA) activity of HN, also destroyed its IFN-α inducing activity; (2) DNA transfectants expressing HN mutant molecules with greatly reduced NA but not HAd activity induced IFN-α (100).
In another study, a serine at position 200 of the HN protein of NDV was reported to be important for functional activities (101). In this study, distinct HN mutants were generated by site-directed mutagenesis and tested to define amino acids responsible for functional activity of HN proteins (101). Substitution of serine 200 by proline abrogated HN expression and its HAd and NA activity (101). Molecular modeling revealed that proline 200 in HN influences flexibility of a loop near the entrance to the neuraminidase active site, a function that may be crucial for this viral protein (101).
Paracrine stimulation of IFN-α responses in human PBMC could be induced either by BHK cell surface exposed viral HN protein or by cytoplasmic viral RNA (44). There are thus two ways to induce innate immune responses in PBMC. We used two replicon systems, which are based respectively on DNA or RNA of Semliki forest virus, and transfected these into BHK cells that do not produce IFN-α (44). HN-expressing BHK cells or BHK cells with cytoplasmic danger signals (as dsRNA replicative intermediates) induced comparable IFN-α responses. These observations highlight two ways of IFN induction, which, additively, may explain the high interferogenic capacity of NDV as a virus (44). All of these results clarify molecular mechanisms involved in pattern recognition during innate immune responses.
The role of type I interferon in the induction of an interferon response is reinforced by its function as link between innate and adaptive immunity (102, 103), as it is shown in Fig. 6 . The innate immune response does not only provide the first line of defense against danger, but it also instructs the adaptive immune system to mount a response (104). Finally, the proinflammatory context procured by the addition of the virus NDV may interfere with the induction mechanism of tolerance as it has been shown with a T cell clone in vitro (70).
Primordial role of interferon-α induced by NDV at the interface of the innate and adaptive immunity. The interaction of NDV infected tumor cells with the innate immunity leads to the production of high level of interferon-α (especially via the plasmacytoid dendritic cells (PDC)). IFN-α has a central role at the interface between the innate and the adaptive immunity.
In conclusion, NDV has very strong immunostimulatory properties that might be used for the generation of a strong antitumoral immune response.
3. Pharmacokinetics of NDV
Biodistribution of oncolytic NDV was investigated in healthy and tumor-bearing mice after systemic i.v. application (105). DBA/2 mice were injected intravenously with 1,500 HU NDV Italien, a lytic NDV strain, and different organs were harvested at different time points after virus application (105). RNA was isolated and transcribed into cDNA. Quantitative RT-PCR allowed the determination of the amount of M and ß-actin mRNA. The detection limit was 7 copies for the M gene and 32 copies for the ß-actin gene. Virus was detected at 0.5 h after i.v. injection mainly in the lung, blood, liver, and spleen. The amount of virus decreased rapidly over time and reached the detection limit at less than 1 day (blood and thymus), 2 days (kidney), and around 14 days (lung, liver, spleen) (105).
4. Toxicity and Pathogenicity of NDV
NDV has a wide host range with at least 27 of 50 orders of birds susceptible to infection, although there is wide variation in clinical response, even among species of the same genus. NDV is categorized into three pathotypes depending on the severity of the disease that it causes in birds: lentogenic (avirulent), mesogenic (intermediate), and velogenic (virulent) (18). Lentogenic NDV do not cause overt clinical signs in adult birds and are considered of low virulence. Viruses of intermediate virulence that cause respiratory disease, but are not usually fatal, are termed mesogenic. Among the highly virulent velogenic NDV isolates, there are viscerotropic forms marked by lesions of the digestive tract, whereas neurotropic forms are characterized clinically by respiratory and neurological signs.
Lentogenic strains are found to behave as non-lytic virus, and velogenic strains as lytic virus. The more virulent NDV strains have a furin cleavage site in their F protein that allows its activation in a proteolytic environment such as the tumor microenvironment. This allows multicyclic viral replication and cross-infection from one tumor cell to another (as shown in Fig. 3 , right). Thus, the properties of the released progeny virus either to be infectious or noninfectious depends on the virulence of the NDV strain.
Cytopathic effects of lytic NDV strains can be seen by formation of plaques in tumor monolayers (plaque assay, see (105) for an example) or in tissue sections (tissue plaque assay). Hydrophobic fusion peptides within the viral envelope promote syncytium formation between infected tumor cells whereby the virus spreads without an extracellular phase, leaving an oncolytic plaque. The killing potential of lytic NDV strains is remarkable. Such strains have been shown to have a high capacity for killing tumor cells. One infectious particle leads in vitro to the death of approximately 10,000 cancer cells in 2–3 days (106).
5. Human Application and Safety of NDV
There is an extensive safety database for NDV (for review, see (19)). Experience with farmers and laboratory workers infected with NDV show that even the wild-type form produces only minimal disease.
The strains that have been most widely evaluated for the treatment of human neoplasms are those represented by MTH68/H, PV-701, 73-T, and Ulster. The first three are lytic whereas Ulster is non-lytic.
Since the publication of the antineoplastic effect of NDV in 1965 (24, 25, 27), NDV is being tested as oncolytic agent using the following different routes: intratumoral, systemic, and nasal. NDV strain 73-T has been injected intratumorally at a dose of 2.4 to 4 × 1012 infectious units into a patient with a cervical cancer (25). The systemic route is the one developed by different groups with different virus strains: PV701 (Wellstat Biologicals, Gaithersburg, MD, USA) (106), HUJ (Theravir, Jerusalem, Israel) (107), and MTH-68/H (developed by Csatary and coworkers) (see (108) for more details).
NDV strain PV-701 was well tolerated in patients with advanced solid cancers in doses of at least 3 × 109 infectious particles by the i.v. route (109–111). Dose-limiting toxicities included dyspnea, diarrhea, and dehydration. When patients were desensitized with a lower initial dose, the maximum tolerated dose (MTD) was increased tenfold (109).
It is remarkable that systemic applications of very high doses of NDV have been extremely well tolerated. NDV was associated with transient thrombocytopenia and diffuse vascular leak. Of all such treated patients, only one possibly treatment-related death (in a terminal patent) was reported. This death was associated with rapid tumor lysis in the lungs by PV701. This compares favorably with safety problems of other phase I oncology studies.
The MTH-68 virus strain is an attenuated variant that was generated by several passages in chicken embryos of the original Hertfortshire strain of NDV designated Herz’33 (112). Studies have demonstrated a remarkable genetic stability, even after prolonged passage. A significant number of cases of primary and metastatic tumors had a partial or total regression after MTH-68/H was used systemically. Significant changes appeared also in laboratory parameters (tumor markers, liver transaminases, and other enzymes). Remarkable are individual case studies of high-grade gliomas (113–115).
It is interesting to note that NDV was also tested in cancer patients by inhalation. Csatary et al. (116) reported seven responses in 33 patients treated by twice-weekly inhalation of NDV strain MTH-68. More than 4,000 patients have been treated in Hungary by inhalation (117). However, many of these patients received other therapies, rendering the interpretation of the data relative to efficacy and safety unclear (117).
From all of these clinical studies, it comes out that NDV, when applied to humans, usually induces only mild fever for a day or conjunctivitis and that severe adverse effects have not been reported despite applications in several thousands of people during 2 decades in Europe and the USA, explaining the regained interest in NDV as an anticancer reagent (118).
In conclusion, it appears that NDV has advantages as a vector because of its safety and tolerability in cancer patients. This high tolerance might be related to the fact that NDV had no chance to adapt to the mammalian host during evolution.
These observations can be combined with some characteristics related to the biology of the virus. The modular nature of gene transcription, the undetectable rate of recombination, and the lack of a DNA phase in the replication cycle make NDV a suitable candidate for the rational design of a safe attenuated vaccine and gene therapy vector (23). There may be additional in vivo mechanisms, such as cell fusion and syncytium formation, that allow virus escape from neutralizing antibodies. The general human population, however, is seronegative when tested for antibodies against NDV antigens (119, 120). The viral vector is not able to lead to cellular transformation. Finally, a robust virus production and manufacturing system is available.
In conclusion, NDV presents key safety features for development as a replication-competent agent to be used for cancer therapy in humans.
6. Rationale for the Use of NDV in a Cancer Vaccine
Multiple evidence has been provided for the existence of tumor-associated antigens (TAAs) in human cancer (121) and for their recognition by the patients’ own immune system, as demonstrated by the presence of autologous tumor-reactive immune T cells and TILs (122).
For active-specific immunization (ASI) of cancer patients, preferably in the adjuvant situation, we designed and developed the autologous tumor vaccine modified by infection with the NDV strain Ulster (ATV-NDV) (123, 124). Tumor cells are isolated from freshly operated tumor specimens by mechanical dissection and enzymatic dissociation, and enriched by Percoll centrifugation. TILs are then removed by immunomagnetic beads. The tumor cells are then frozen and an ampule that contains 107 cells is thawed for each vaccination. NDV is absorbed to the tumor cells and the cells are then γ-irradiated. The virus-modified tumor vaccine is injected intradermally, thus allowing for virus replication in vivo at the site of vaccine application. Vaccine consisting of tumor cells infected with the Ulster strain of NDV, which is non-lytic, should remain in the body long enough to generate effective immune responses, which are mostly based on T cell-mediated immunity. Viral replication in the tumor cells takes approximately 6–40 h (30), a time sufficient to generate DTH skin responses, which are dependent on antigen-specific memory T cells.
The rationale of this vaccine is to link multiple TAAs from individual patient-derived tumor cells with multiple danger signals (DS) derived from virus infection (e.g., dsRNA, HN, IFN-α) (see Fig. 7 ). Note:
-
1.
The use of autologous tumor cells enables a close match between the TAAs of the vaccine and those of the patient’s tumor and includes common and individually unique TAAs. Nowadays, we know that such unique TAAs are the results of somatic point mutations occurring in many different proteins expressed by tumor cells (125) and, therefore, they represent the only true, tumor-specific antigens that are not expressed by any normal tissue. Other possible but less frequent mechanisms for generation of these TAAs, such as alterations in RNA splicing, have been reported (126, 127). An important additional feature of unique TAAs is their potential resistance to immunoselection in cases in which the mutated protein is crucial to the oncogenic process and thus indispensable for maintaining the neoplastic state or because it is functionally involved in fundamental pathways of cell survival. These unique TAAs may be most important for the induction of tumor-specific protective immunity, also because T cells with high affinity receptors to these antigens should not have been affected by tolerance mechanisms in the host.
-
2.
It is well established that tumor antigenicity is not equal to tumor immunogenicity. Our approach using a non-lytic, nonvirulent strain of NDV (Ulster) to infect tumor cells increases the immunological properties of these tumor cells without lysing them (10, 11). Such modification of the tumor cells by the addition of NDV allows, as described above, activation of multiple innate immune responses (through NK cells, monocytes, and DCs) as well as adaptive immune responses (implicating CD4 and CD8 T cells, mostly preexisting memory T cells).
Rationale for combining TAAs with danger signals in a tumor vaccine. An autologous tumor vaccine modified by infection with NDV (ATV-NDV) consists of tumor cells that are obtained by short in vitro culture of tumor cells from resected tumors of patients. For vaccine production, the cells are infected with the non-lytic NDV strain Ulster and inactivated by γ-irradiation. The use of autologous tumor cells allows the inclusion of individually unique TAAs and also the restimulation of a broad polyclonal antitumor memory response. The modification of the tumor cells by infection with NDV introduced danger signals, co-stimulatory signals, and immunostimulatory properties. For vaccination, 10 million ATV-NDV cells are injected intradermally.
6.1 Preclinical Work in Animal Models
The strategy of development of this tumor vaccine is based on our experience over many years with immunotherapy studies in animal tumor models (123). Approximately 20 years ago, we started this work in the murine ESb lymphoma animal tumor model. The ESb lymphoma is very aggressive and metastasizes to visceral organs, in particular to the liver, killing syngeneic DBA/2 mice within approximately 12 days. After operation of a small intradermal transplanted tumor (5-mm diameter), animals succumb from the outgrowth of metastases derived from disseminated tumor cells and micrometastases at the time of operation. Postoperative vaccination with irradiated ESb vaccine cells infected with NDV—but not with uninfected vaccine cells—was able to protect approximately 50% of the mice (53). These long-term surviving mice developed tumor-specific protective immunity as revealed by challenging them with ESb and unrelated tumor cells (128).
The effectiveness of this ASI approach was thereafter confirmed in other metastasizing animal tumors, such as murine B16 melanoma (129), 3LL Lewis lung carcinoma (130), and guinea pig L10 hepatocarcinoma (131) (for review see (132)).
6.2 Preclinical Work with Human Cells
By infecting patient-derived tumor cells with NDV, we developed the tumor vaccine ATV-NDV. A large variety of human tumor cells was shown by flow cytometry analysis to be efficiently infectible by NDV. More than 400 noncultured freshly separated tumor cells, as they are used for the production of the ATV-NDV vaccine, were found to be infected by NDV (30). Viral replication was observed to be independent of host cell proliferation, and γ-irradiation did not affect viral replication. All of these properties make NDV a suitable agent for modification of noncultured freshly isolated and γ-irradiated patient-derived tumor cells as well as of cultured tumor cell lines (133). Human tumor cell infection was found to be an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties (133).
Figure 8 shows a diagram recapitulating the immunological consequences of infection of a tumor cell by NDV during the elaboration of the ATV-NDV tumor vaccine. Most of these mechanisms have already been mentioned in Chapter III. The NDV-induced cellular changes—induction of IFN-α, IFN-β, chemokines (IP-10, RANTES), apoptosis, but also enhancement of MHC expression and of adhesion molecules (71, 72)—affect the microenvironment between antigen-presenting cells (APC) and T cells at the application site of the vaccine. This also has effects on components of the innate system and on components of the adaptive immune system, as shown above (see Fig. 8 and see review (134)).
Modification of the properties of tumor cells by NDV infection. The replication of NDV in tumor cells leads to the modification of the tumor cells properties and consequently to their interactions with innate (left) and adaptive (right) immunity. Induction of chemokines (such as RANTES, IP-10) and induction of apoptosis by the viral replication stimulate antitumor innate immunity. Viral infection of the tumor cells also leads to enhancement of antigen presentation (notably by upregulation of the expression of MHC molecules) and to enhanced adhesion and co-stimulation (as observed by the increased expression of intercellular adhesion molecule (ICAM)-1 and LFA-3 molecules. Interferon induction by the infected tumor cells also plays an important role in the induction of an antitumor immune response.
Figure 9a shows the principle of the in vitro tumor neutralization assay (TNA) and representative results obtained in this assay with the virus-modified vaccine. PBMC, which were co-incubated with such a tumor vaccine, were able to induce variable degrees of antitumor activity against monolayers of human breast carcinoma or stomach carcinoma cells (see Fig. 9b and c , respectively).
Tumor neutralization assay: principle (A) and results (B and C). A: Effector cells (PBMC) from a healthy donor are incubated for 5–6 days on top of a tumor cell monolayer with virus-infected tumor vaccine from an established tumor cell line. Activation of effector cells leads to tumor cell killing and to the inhibition of tumor cell growth. This can be quantified by staining the remaining live cells with the chemical reagent MTS. Graphs on the right side show representative data of tumor growth inhibition of mammary carcinoma (B) and stomach carcinoma (C) cells achieved with PBMC from four different healthy donors (for more details, see (147)).
6.3 Clinical Data
A certain number of clinical studies that are based on ASI with the vaccine ATV-NDV have been performed (reviewed in (132)). First, it is important to mention that the intradermal vaccinations were well tolerated and could be repeated many times without causing serious problems (Tables 1 and 2 ).
Our first study of postoperative ASI was performed in curatively resected colorectal cancer patients with the tumor cell vaccine ATV-NDV. ASI was performed in a phase I study in 20 colorectal cancer patients. After mechanical and enzymatic dissociation of tumor tissue, an average of 5 × 107 cells/g tissue was obtained. According to trypan blue dye exclusion assay, the average viability was 72%. After γ-irradiation (200 Gy), the inactivation of proliferative activity of the cells could be demonstrated by the absence of incorporation of tritium-labeled thymidine. The cells were still metabolically active as shown by uptake of tritium-labeled uridine and of a mixture of tritium-labeled amino acids. The successful modification of tumor cells with the Ulster strain of NDV has be shown by electron microscopy.
Viability of the irradiated vaccine was observed to be important not only for CTL activation (135) but also for clinical efficacy (136). Antitumor memory T cells from cancer patients could be activated and their frequency increased by antitumor vaccination with ATV-NDV as revealed by augmentation of antitumor memory DTH and by ELISPOT assays (137). DTH responses to TAA could be distinguished from responses to recall antigens and from autoimmune responses. Reactivity to NDV alone was either not seen or was weak in a minority of patients. Similar distinctive responses were obtained with ELISPOT, the memory test in vitro, which is based on single-cell IFN-γ production.
Several cell and virus concentrations were tested in each patient. Sixteen patients responded with a DTH skin reaction to the vaccine. The best DTH reaction was obtained using a vaccine composed of 107 tumor cells and 32 HU NDV (median induration of 8 mm). DTH responses to the vaccine increased throughout repeated vaccinations. Of ten patients tested for DTH response with normal colon mucosa, four responded with a median induration of 3.5 mm (138).
A first evaluation of clinical response revealed the following. After a follow-up of at least 18 months, 61% of the vaccinated patients developed tumor recurrence in comparison with 87% of a matched control group from the same institution that had been only surgically treated (139).
In another clinical study, 16 patients with primary operated colorectal carcinoma (Dukes’ stage B2, C, or D) were treated with ATV-NDV up to four times at 10-day intervals. In 11 patients, an increasing reactivity against the vaccine was observed. A challenge test using autologous tumor cells without NDV after the vaccination cycle revealed a specific antitumor sensibilization in 12 patients. The DTH response was not caused by bacterial contamination or sensibility to the virus. Histological examination of the vaccination site showed a dense infiltration of predominantly helper T lymphocytes. It was concluded that, in most of the patients treated, ASI led to a specific antitumor response (140, 141).
Of 57 patients with operated locally advanced colorectal carcinoma who received ASI, 48 were treated by virus-infected ATV (ATV-NDV) and 9 were treated with Bacille Calmette–Guérin (BCG) admixed autologous vaccine (ATV/BCG). The application of the ATV-NDV vaccine was associated with only mild side effects whereas the ATV/BCG vaccine led to long-lasting ulcers and to more serious side effects. The 2-year survival rate with ATV-NDV was 97.9%, whereas the survival rate with ATV/BCG was 66.7%. The mean survival of 661 patients from a historical control was 73.8% (see Table 1 ). These data suggest that the type and quality of the tumor vaccine for ASI treatment is important (142).
Another clinical study was performed in patients with advanced renal cell carcinoma. They were treated sequentially with the autologous tumor vaccine ATV-NDV and subcutaneous administration of IL-2 and IFN-α2b. Thirty patients with advanced renal cell carcinoma (RCC) were entered after nephrectomy into the protocol. Three of the patients exhibited a complete response and four displayed partial remission, seven showed stable disease during 1–18 months (median, 5 months), and progression was seen in nine. It is concluded that vaccination combined with cytokines can induce regressions in patients with advanced RCC and that, even in nonresponding patients, a more favorable course of the disease can be achieved.
After a longer follow-up, we reported on tumor response and 4-year survival data of patients with advanced renal carcinoma treated with autologous tumor vaccine and subcutaneous recombinant IL-2 and IFN-α2b (143). Of the 40 evaluable RCC patients, 5 exhibited a complete response (CR), 6 displayed a partial remission (PR), 12 showed stable disease (SD) with a median survival of 25 months, and 17 had tumor progression. Twenty-three (57.5%) of the 40 patients (either with CR, PR, or SD) appeared to have a significant survival advantage compared with patients with progressive disease during the treatment period and compared with a historic reference group (143).
Quality and efficacy criteria were evaluated for an ATV-NDV vaccine by analyzing three independent cohorts, (a), (b), and (c), of breast and ovarian cancer patients vaccinated between 1991 and 1995. Cohort (a) included 63 patients with primary breast cancer, cohort (b) included 27 patients with metastatic pretreated breast cancer, and cohort (c) included 31 patients with metastatic pretreated ovarian cancer. We retrospectively hypothesized that an immunogenic vaccine should contain at least 1.5 × 106 viable tumor cells and viability should be at least 33%. Each cohort was thus divided into two groups: one that received vaccine type A fulfilling both criteria; and the other, type B, missing one or both criteria. Prognostic factors were well balanced between A and B in cohorts (a) and (c). In cohort (a), there was a benefit in survival (P = 0.026) for type A. The relative risk of dying in the group that received type A as compared with type B was 0.2 (univariate Cox model). In cohort (c), like in cohort (a), the survival benefit for vaccine type A may be ascribed to the vaccine quality, because prognostic factors were not biased. This could imply clinical effectivity in breast and ovarian cancer with ATV-NDV high-quality vaccine (144).
The outcome of clinical trials of antitumor vaccination with ATV-NDV was reviewed 2005 (132). This review includes follow-up evaluations of earlier published phase II studies. Tables 1 and 2 summarize, respectively, the 2-year and 5-year survival rates obtained in the studies. In the ASI study of locally advanced primary breast cancer, the follow-up revealed a 5-year survival rate that was 36% higher in group A (high-quality vaccine) than in group B (low-quality vaccine) (123, 132). In the colorectal carcinoma study, there was a 25% increase in the 5-year survival rate in a group of 20 patients who received a high-quality ATV-NDV vaccine (123, 132; see Table 2 ).
All of these studies led to the following conclusions:
-
1.
Postoperative vaccination with ATV-NDV derived from fresh operation specimens appears to be feasible and safe in colorectal carcinoma, breast, and ovarian cancer, and advanced renal cell carcinoma.
-
2.
Tumor cell number and cell viability appear of importance for vaccine quality.
-
3.
Application of high-quality ATV-NDV vaccine appears to improve the prognosis for survival of vaccinated patients.
Another clinical study of antitumor vaccination was performed with patients suffering from glioblastoma multiforme (124). This was a pilot study to assess feasibility, safety, and clinical benefit. In a nonrandomized study, 23 patients were vaccinated postoperatively and results were compared with nonvaccinated control subjects (n = 87). Vaccine was prepared from the patient’s tumor cell cultures by infection with NDV followed by γ-irradiation and applied up to eight times. Establishment of tumor cell cultures was successful in approximately 90% of patients. After vaccination, no severe side effects were observed. The median progression-free survival of vaccinated patients was 40 weeks (versus 26 weeks in control subjects; P = 0.024) and the median overall survival of vaccinated patients was 100 weeks (versus 49 weeks in control subjects, P < 0.001). In the vaccinated group, immune monitoring revealed significant increases of DTH reactivity, of the number of tumor-reactive memory T cells, and of the numbers of CD8 tumor-infiltrating T lymphocytes in secondary tumors (124).
We also performed antitumor vaccination in patients with head and neck squamous cell carcinomas (HNSCC) (145). In a nonrandomized pilot study, 20 patients were vaccinated postoperatively with ATV-NDV. Establishment of patient-derived tumor cell cultures was successful in approximately 80% of the cases. After vaccination, no severe side effects were observed. Percentages of survival of vaccinated patients with stage III and stage IV tumors (n = 18) were 61% at 5 years (145, see Table 2 ). Immune monitoring revealed significant increases of antitumor DTH reactivity and, in a significant proportion of vaccinated patients, the presence of tumor-reactive T cells in the peripheral blood even 5–7 years after the last vaccination (145). Postoperative vaccination with ATV-NDV may improve the prognosis of HNSCC patients with advanced tumors (145).
In conclusion, the clinical studies performed demonstrate postoperative vaccinations with ATV-NDV derived from cell culture to be feasible and safe. Although they were not obtained in randomized studies, they show a strong tendency to improvement of survival upon vaccination with ATV-NDV. This could be substantiated by the observed vaccination-induced improvement of the antitumor immune responses. A positive correlation between increase in DTH responsiveness to ATV-NDV and prognosis was observed (137).
All of the clinical data presented are summarized in Tables 1 and 2 . Table 3 lists some possible explanations for the results of such a vaccination strategy.
7. Future Developments Relating to NDV
7.1 Combination of ATV-NDV Vaccine with Virus-Specific Bispecific scFv Antibodies
The aim is to develop T cell co-stimulatory molecules that are broadly applicable to augment antitumor immune responses upon application of the ATV-NDV tumor vaccine to cancer patients. To this end, we generated recombinant bispecific single-chain antibodies with a dual binding specificity. One is directed toward the CD3 or the CD28 antigen, which is expressed on human T cells. The other one is specific for the viral target molecule HN, which is expressed at the surface of ATV-NDV (146). These bispecific molecules are named, respectively, bsHN-CD3 and bsHN-CD28. By redirecting unstimulated human T cells against HN-expressing NDV-infected tumor cells, the bispecific molecule bsHN-CD3 were found to crosslink effector and target cells and to rapidly induce cytotoxicity at nanomolar concentrations (146). Figure 10 shows a scheme of the new immunostimulatory fusion proteins and their way of function in combination with the vaccine ATV-NDV. The bsHN-CD3 protein enhanced the signal 1. The bsHN-CD28 molecule exerted T cell co-stimulatory function and led to increase of signal 2. Maximal T cell activation was achieved with tumor cells infected by NDV and modified with both new stimulatory molecules at the same time (146). This was revealed by T cell proliferation; upregulation of CD69 and CD25; and by release of cytokines, interferons, and chemokines (146). We concluded that these newly developed molecules combine high efficiency with specificity and safety (146).
Second-generation ATV-NDV tumor vaccine loaded with new immunostimulatory fusion proteins anchored to HN. The ATV-NDV vaccine interacts with T cells via the expression of the tumor antigen (TA) in the context of MHC restriction (signal 1) but also via the expression of the viral HN protein (signal 2). The released IFN-α provides a survival signal to memory T cells represented as signal 3. The addition of the bispecific protein bsHN-CD3 enhances signal 1. The bispecific protein bsHN-CD28 leads to an increased signal 2 (co-stimulation). This optimized tumor vaccine has been showed in in vitro TNA assays to augment T cell antitumor activity as shown in Fig. 11 TAA, tumor associated antigen; MHC, major histocompatibility complex; TCR, T cell receptor; IFN-R, interferon type I receptor; PDC, plasmacytoid dendritic cells.
We also observed that, when bound via one arm to the human tumor-NDV vaccine, the new molecules caused a polyclonal T cell response and could overcome the potential various T cell evasion mechanisms of tumor cells. In the TNA assay presented in Fig. 9 , PBMC or purified T cells that were activated with breast or stomach carcinoma vaccine infected by NDV and modified with bsHN-CD3 and bsHN-CD28 were able to destroy completely monolayers of such carcinoma cells ((147), see Fig. 11 ). Nontumor target cells appeared to be much less sensitive to the activated effector cells (147).
Results of tumor neutralization assays showing the superiority of the vaccine ATV-NDV loaded with the two bispecific antibodies bsHN-CD3 and bsHN-CD28. The TNA assays were performed as described in Fig. 9a . Tumor growth inhibition of a mammary carcinoma (A) and a stomach carcinoma (B) cell line with effector cells (from four different healthy donors) activated with tumor vaccine generated from the corresponding mammary carcinoma or the stomach carcinoma cell lines. Notice the increase of antitumor activity upon addition of the bispecific antibody fusion proteins bsHNxCD3, recombinant bispecific single-chain antibodies with a binding specificity for the CD3 on human T cells and also for the viral target molecule HN, which is expressed at the surface of ATV-NDV; bsHNxCD28, recombinant bispecific single-chain antibodies with a binding specificity for the CD28 antigen on human T cells and also for the viral target molecule HN, which is expressed at the surface of ATV-NDV.
These new bioactive molecules can, in combination with an established tumor vaccine—but not by themselves alone—greatly augment antitumor activity of T cells from healthy donors and cancer patients (58). This second-generation vaccine is far superior to the first-generation vaccine ATV-NDV in that:
-
1.
It is more immunogenic;
-
2.
It can activate naïve T cells;
-
3.
It is less dependent on TAA recognition; and, consequently,
-
4.
It is also expected to be less affected by tumor immune escape mechanisms.
7.2 Bispecific Antibodies for Tumor-Specific Retargeting of NDV
We also developed a novel strategy to target recombinant NDV to tumor cells for gene therapy. For this purpose, we modified the binding properties of the virus by attaching adaptor molecules that neutralize the virus and restore binding property to the viral particles. As a proof of principle, we constructed and used the recombinant molecule anti-HNxIL-2, which contains a scFv antibody cloned from a neutralizing HN-specific hybridoma linked to the human cytokine IL-2 (148).We observed that modifying the virus with such a bispecific fusion protein allowed virus receptor-independent tumor cell binding and gene transfer (148). Hemadsorption (HAd) and neuraminidase (NA) activity of HN of NDV was shown to be blocked by anti-HNxIL-2 (149). A recombinant NDV expressing enhanced green fluorescence protein (EGFP) was applied to show the expression of foreign genes in virus infected tumor cells. Selective virus entry to IL-2 receptor-bearing tumor cells was observed in a mixture of Jurkat and MT-2 (IL-2 receptor [IL-2R]-positive) tumor cells (148). Retargeted virus binding to IL-2R-expressing tumor cells was not sufficient for the process of virus infection. This required in addition membrane fusion via the viral F protein.
The purpose of another study was to investigate the specificity and efficiency of gene delivery to tumor cells in vivo via anti-HNxIL-2 modified NDV virus, 24 h after intratumoral delivery (150). Direct fluorescence microscopy and immunohistology revealed viral replication in target-positive tumor tissue, resulting in much more EGFP expression than in target-negative tumor tissue (150). Biodistribution studies showed that EGFP transgene delivery was reduced by 35–100% in liver, spleen, kidney, lung, and thymus, whereas 98% of the transgene was delivered to IL-2R-positive tumors (150).
Tumor therapy experiments revealed that the side effects on liver and kidney induced by systemic virus application were greatly reduced by the adaptor protein whereas the antitumor effects were mostly undiminished (105).
In conclusion, the modification of recombinant NDV with a bispecific molecule appeared to be a novel and safe strategy for selective gene transfer to targeted tumor cells (149). The demonstration of significant systemic antitumor activity of this retargeted viral vector suggests potential for improvement by inclusion of one or more therapeutic genes.
7.3 Use of Recombinant NDV as a Vector for Gene Therapy
Through the use of recombinant DNA technology, it is now possible to generate recombinant strains from nonsegmented negative-strand RNA viruses (118, 150–153). Reverse genetic systems are now established in different laboratories to recover recombinant NDV (recNDV) entirely from cloned cDNA (154, 155). This unique molecular genetic methodology provides the means to investigate the functions of various virus-encoded genes in more detail and to lead to extraordinary advances in understanding their biology. As example, generation of recNDV with reduced V protein expression and a lack of pathogenicity for chicken embryos (156) showed the importance of V protein in the viral pathogenicity in chickens.
In addition, the development of such viruses allows the use of these viruses to express heterologous foreign genes. In this way, NDV could be more specifically designed and its efficacy enhanced (as described recently for oncolytic (157) and vaccination (31) purposes). Certain characteristics of NDV suggest that recNDV expressing a foreign protein would be a very good vaccine candidate to deliver protective antigens of respiratory disease pathogens because NDV naturally infects via respiratory and alimentary tract mucosal surfaces.
A gene encoding a foreign protein inserted in the NDV genome is expressed together with only a few NDV proteins. In contrast, Pox and Herpes virus vectors express a large number of additional proteins from their large genomes.
In a recent study, the gene encoding granulocyte/macrophage colony-stimulating factor (GM-CSF) was inserted as an additional transcription unit at two different positions of the NDV genome. The recNDV with the strongest production of the gene product, which corresponds to the insertion of the GM-CSF gene before the NP gene, was then selected for further study (see Fig. 12 ). This observation is in concordance with previous data (158, 159) showing an effect of the genomic location of the insertion of the foreign gene on its gene expression. The gene product was biologically active and stable (31). Tumor vaccine cells infected with recNDV with GM-CSF as incorporated gene (rec[GM-CSF]) stimulated human PBMC to exert antitumor bystander effects in vitro in the tumor neutralization assay (TNA), which is described in Fig. 9a . These effects were significantly increased when compared with vaccine infected by recNDV without an inserted gene (rec(−)) (31). Finer analysis showed that rec(GM-CSF) led to a much higher IFN-α response than rec(−) when added as virus or as virus-modified vaccine to PBMC (31). Furthermore, two distinct cell types, plasmacytoid DCs (pDC) and monocytes, were shown to contribute to the augmented IFN-α response of PBMC (31). We concluded that the already inherent antineoplastic and immunostimulatory properties of NDV could be further augmented by the introduction of the therapeutic gene as a proof of principle. We observed also that GM-CSF initiated, in combination with the virus, a broad cascade of immunological effects in the microenvironment of the tumor vaccine (Fig. 12 ) (31).
Incorporation of GM-CSF as therapeutic gene into recombinant NDV. Using reverse genetics, the GM-CSF gene has been inserted before the NP gene within the viral genome. Each viral gene is separated by a junction that consist of three elements, known as gene-end (GE), intergenic (IG), and gene start (GS) sequences. These sequences have been taken into consideration for the insertion of the therapeutic gene. The use of such recombinant virus (rec[GM-CSF]) has been shown to induce an increased antitumor activity when compared with the recombinant virus having no inserted therapeutic gene. The production of GM-CSF after infection of the irradiated tumor cell vaccine was shown to synergize with the virus effect and to lead to an increased production of interferon-α in vitro upon co-incubation with human PBMC (for more details, see (31)). DC, dendritic cells; GM-CSF, growth macrophage colony-stimulating factor; Rec(GM-CSF), recombinant NDV with GM-CSF as insert
Further recent studies have confirmed the potential of recombinant NDV as a vaccine vector. The expression levels of foreign proteins (e.g., chloramphenicol acetyltransferase [CAT] (157) or influenza virus hemagglutinin (160)) are high and the foreign genes are very stable after many passages in vitro and in vivo. RecNDV, which was modified by the insertion of a gene cassette encoding the human parainfluenza virus type 3 (HPIV3) HN protein between the P and M genes, was shown to induce a high serum antibody response to the foreign HN protein induced by the first immunization, although somewhat less than that observed after a wild-type HPIV3 infection (161). However, the titer after the second immunization exceeded the titer observed with HPIV3 infection, even though HPIV3 replicates much more efficiently than NDV in these animals (161). Furthermore, recNDV completely protected chickens and mice from lethal challenges of homologous and heterologous H5N1 avian influenza viruses (162). Recombinant Newcastle disease virus (NDV) capsids displaying the enterovirus 71 VP1 fragment was shown also to be able to induce a strong immune response in rabbits (163). NDV expressing the H5 hemagglutinin gene protects chickens against Newcastle disease but also against avian influenza (164). A recNDV expressing the VP2 protein of infectious bursal disease virus (IBDV) has also been shown to protect against NDV and IBDV (165). Similar results have been obtained with recombinant NDV engineered to express the SARS-CoV spike S glycoprotein, which protected African green monkeys against challenge with a high dose of SARS-CoV virus (32). It was therefore suggested that NDV might be a perfect vector for intranasal immunization against newly emerging pathogens (32).
The technology of reverse genetics can be used to modify the binding properties of NDV (as it has been done for measles virus) or its conditional replication. For example, virologists in the Virginia–Maryland Regional College of Veterinary Medicine at Virginia Tech are looking at how a genetically modified variant of NDV can be used to treat human prostate cancer. Dr. Elankurmaran Subbiah and coworkers altered the fusion protein of NDV to replicate only in the presence of prostate specific antigen (PSA) (not published).
It is also possible to engineer recNDV to allow retargeting to tumor by cloning fragments of antibodies into the viral F or HN proteins. RecNDV may also be engineered to secrete full intact antibodies if respective heavy and light chains of IgG from a hybridoma are incorporated in the viral genome. A recNDV with tumor-selective binding properties and secreting antibody would allow a high antibody concentration in the tumor microenvironment to be reached, and allow a deeper penetration of antibodies into the tissue of solid tumors. This could then avoid side effects observed with systemic application of antibody. The insertion of genes into NDV for drug activation could be of benefit when associated with the application of an anticancer drug. The enveloped, negative-stranded RNA viruses do not have the packaging constraints that encapsulated DNA viruses have. Nevertheless, it remains to be determined how many transgenes may be expressed and which maximal genome size may be tolerated by NDV.
In conclusion, NDV appears to be a very attractive vector for virus therapy, immune therapy, and also for gene therapy of cancer.
References
Aghi M. and Martzua R.L. (2005). Oncolytic viral therapies—the clinical experience. Oncogene 24: 7802–7815.
Chlichlia K., Schirrmacher V. and Sandaltzopoulos R. (2005). Cancer immunotherapy: battling tumors with gene vaccines. Curr. Med. Chem. Anti-inflammatory Anti-allergy Agents 4: 353–365.
Schirrmacher V. (2005). T cell mediated immunotherapy of Metastases: State of the art in (2005). Expert Opin. Biol. Ther. 4 (8): 1051–1068.
Sinkovics J. and Horvatz J. (1993) New developments in the virus therapy of cancer: a historical review. Intervirology 36: 193–214.
Asada T. (1974) Treatment of human cancer with mumps virus. Cancer 34 (6):1907–1928.
Shimizu Y., Hasumi K., Okudaira Y., Yamanishi K. and Takahashi M. (1988). Immunotherapy of advanced gynecologic cancer patients utilizing mumps virus. Cancer Detect. Prev. 12: 487–495.
Csatary L.K., Eckhard S., Bukosza I., Czegledi F., Fenyvesi. C., Gergely P., Bodey B. and Csatary C.M. (1993) Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 17: 6 19–627.
Reichard M.W., Lorence, R.M. and Cascino C.J. (1992) Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 52: 448–453.
Sinkovics J.G. (1991) Viral oncolysates as human cancer vaccines. Int. Rev. Immunol. 7: 259–287.
Schirrmacher V., Ahlert I., Heicappell R., Appelhans B. and Von Hoegen P. (1986) Successful application of non-oncogenic viruses for antimetastatic cancer immunotherapy. Cancer Rev. 5:19–49.
Heicappell R., Schirrmacher V., von Hoegen P., Ahlert T. and Appelhans B. (1986). Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I: parameters for optimal therapeutic effects. Int. J. Cancer 37: 569–577.
Lorence, R.M., Reichard K.W., Katubig B.B., Reyes H.M., Phuangsab H., Mitchell B. R., Cascino J., Walter R.J. and Peeples M.E. (1994) Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J. Ntl Cancer Inst. (Bethesda) 86: 1228–1233.
Umansky V., Shatrov V.A., Lehmann V. and Schirrmacher V. (1996) Induction of NO synthesis in macrophages by Newcastle disease virus is associated with activation of nuclear factor-kappa B. Int. Immunol. 8 (4): 491–498.
Cassel W.A. and Murray D.R. (1992) A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 9 (4):169–71.
Russell S.J. (2002) RNA viruses as virotherapy agents. Cancer Gene Ther. 9: 961–966.
Stoidl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N. and Bell J. (2000) Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nature Med. 6 (7): 821–825.
Kasuya H., Takeda S., Shimoyama S., Shikano T., Nomura N., Kanazumi N., Nomoto S., Sugimoto H. and Nakao A. (2007) Oncolytic virus therapy—foreword. Curr Cancer Drug Targets 7 (2): 123–125.
Alexander D.J. (1997). Newcastle disease and other Paramyxoviridae infections. In: Diseases of Poultry (Calnek, B.W., Barnes H.J., Beard C.W., McDougald L. and Saif J.Y.M. eds.), 10th ed. Lowa State University, Ames, IA, pp. 541–569.
Lorence R.M., Roberts M.S., Groene W.S. and Rabin H. (2003) Replication-competent, oncolytic Newcastle disease virus for cancer therapy. In: Replication-Competent Viruses for Cancer Therapy. (Hernaiz Driever P., Rabkin S.D., eds.), Collection: Monographs in Virology Basel, Karger, vol. 22, pp. 160–182.
Alexander D.J. (1988) Historical aspects. In: Newcastle Disease. (Alexander D.J. (ed.) Kluwer, Boston, pp. 1–10.
Doyle T.M. (1927) A hitherto unrecorded disease of fowls due to a filter-passing virus. J Comp Pathol Ther 40: 144–169.
de Leeuw O. and Peeters B. (1999) Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae. J. Gen. Virol. 80 (Pt 1): 131–136.
Lamb RA., Parks G.D. (2007) Paramyxoviridae: their viruses and their replication. In Fields Virology. Fifth edition. (Knipe D.M. and Howley P.M. eds). Wolters Kluwer /Lippincott Williams & Wilkins, pp. 1449–1496.
Wheelock E.F., Dingle J.H. (1964) Observations on the repeated administration of viruses to a patient with acute leukaemia. A preliminary report. N. Engl. J. Med. 24: 271:645–651.
Cassel W.A., Garrett R.E. (1965) Newcastle Disease Virus as an antineoplastic agent. Cancer. 18: 863–868.
Csatary L.K. (1971) Viruses in the treatment of cancer. Lancet 2 (7728): 825.
Sinkovics J.G., Horvath J.C. (eds) (2005) Viral therapy of cancer, Marcel Dekker, New York.
http://www.cancer.gov/cancertopics/pdq/cam/NDV/HealthProfessional/
Sinkovics J.G. and Horvath J.C. (2000). Newcastle Disease Viurs (NDV): brief history of its oncolytic strains. J. Clin. Virol. 16 (1): 1–15.
Schirrmacher V., Haas C., Bonifer R., Ahlert T., Gerhards R. and Ertel C. (1999). Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle Disease Virus. Gene Ther. 6: 63–73.
Janke M., Peeters B., de Leeuw O., Moorman R., Arnold A., Fournier P., Schirrmacher V. (2007). Recombinant Newcastle Disease Virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Ther. 14(23): 1639–1649.
DiNapoli J.M., Kotelkin A., Yang L., Elankumaran S., Murphy B.R., Samal S.K., Collins P.L. and Bukreyev A. (2007). Newcastle Disease Virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. USA 104 (23): 9788–9793.
Nagai Y., Hamaguchi M., Toyoda T. (1989) Molecular biology of Newcastle disease virus. Prog Vet Microbiol Immunol. 5: 16–64.
Yusoff K., Tan W.S. (2001) Newcastle disease virus: macromolecules and opportunities. Avian Pathol. 30: 439–455.
Calain P., Roux L. (1993) The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67 (8): 4822–4830.
Peeters B.P., Gruijthuijsen Y.K., de Leeuw O.S., Gielkens A.L. (2000) Genome replication of Newcastle disease virus: involvement of the rule-of-six. Arch. Virol. 145 (9): 1829–1845.
Lamb R.A., Paterson R.G., Jardetzky T.S. (2006) Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 344 (1): 30–37.
Suzuki Y., Suzuki T., Matsunaga M., Matsumoto M..J. (1985) Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. Biochem (Tokyo) 97 (4): 1189–1199.
Ferreira L., Villar E. and Munoz-Barroso I. (2004). Gangliosides and N-glycoproteins function as Newcastle Disease Virus receptors. Int. J. Biochem. Cell Biol. 36: 2344–2356.
Villar E. and Barroso I.M. (2006) Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj J. 23 (1–2): 5–17.
Cantin C., Holguera J., Ferreira L., Villar E. and Munoz-Barroso I. (2007). Newcastle Disease Virus may enter cells by caveolae-mediated endocytosis. J. Gen. Virol. 88: 559–569.
Laliberte J.P., McGinnes L.W., Peeples M.E., Morrison T.G. (2006). Integrity of membrane lipid rafts is necessary for the ordered assembly and release of infectious Newcastle Disease Virus particles. J. Virol. 80 (21): 10652–10662.
Pantua H.D., McGinnes L.W., Peeples M.E., Morrison T.G. (2006). Requirements for the assembly and release of Newcastle Disease Virus-like particles. J. Virol. 80 (22): 11062–11075.
Fournier P., Zeng J. and Schirrmacher V. (2003). Two ways to induce innate immune responses in human PBMCs: Paracrine stimulation of IFN-α responses by viral protein or dsRNA. Int. J. Oncol. 23: 673–680.
Taniguchi T. and Takaoka A. (2002). The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14: 111–116.
Sadler A.J. and Williams B.R. (2007) Structure and function of the protein kinase R. Curr Top Microbiol Immunol. 316: 253–292.
Fiola C., Peeters B., Fournier P., Arnold A., Bucur M. and Schirrmacher V. (2006) Tumor selective replication of Newcastle Disease Virus: association with defects of tumor cells in antiviral defence. Int. J. Cancer 15, 119 (2): 328–338.
Haller O., Kochs G. and Weber F. (2007) Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 18 (5–6): 425–433.
Malathi K., Dong B., Gale M. and Silverman R.H. (2007) Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 446: 816–819.
Ahlert T., Schirrmacher V. (1990) Isolation of a human melanoma adapted Newcastle disease virus mutant with highly selective replication patterns. Cancer Res. 50 (18): 5962–5968.
Fábián U., Csatary C., Szeberényi J. and Csatary L.K. (2007). p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle Disease Virus strain in tumor cell lines. J. Virol. 81 (6): 2817–2830.
Elankumaran S., Rockemann D. and Samal S.K. (2006). Newcastle Disease Virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 80 (15): 7522–7534.
Liu T.C. and Kirn D. (2007) Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 67 (2): 429–432.
Apostolidis L., Schirrmacher V. and Fournier P. (2007). Host mediated anti-tumor effect of oncolytic Newcastle Disease Virus after locoregional application. Int. J. Oncol. 31: 1009–1019.
Ito Y., Nagai Y. and Maeno K. (1982) Interferon production in mouse spleen cells and mouse fibroblasts (L cells) stimulated by various strains of Newcastle disease virus. J. Gen. Virol. 62 (Pt 2): 349–352.
Lorence R.M., Rood P.A. and Kelley K.W. (1988) Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J. Natl. Cancer Inst. 80 (16): 1305–1312.
Aigner M., Fournier P. and Schirrmacher V. (2007) An effective tumor vaccine with optimized costimulation via bi- and trispecific fusion proteins. Int. J. Oncol. (in press).
Sato K., Hida S., Takayanagi H., Yokochi T., Kayagaki N., Takeda K., Yagita H., Okumura K., Tanaka N., Taniguchi T. and Ogasawara K. (2001) Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur. J. Immunol. 31: 31–38.
Schirrmacher V., Bai L., Umansky V., Yu L., Xing Y. and Qian Z. (2000). Newcastle Disease Virus activates macrophages for antitumor activity. Int. J. Oncol. 16: 363–373.
Umansky V., Shatrov V.A., Lehmann V. and Schirrmacher V. (1996). Induction of nitric oxide synthesis in macrophages by Newcastle Disease Virus is associated with activation of nuclear factor-B. Int. Immunol., 8 (4): 491–498.
Washburn B., Weigand M.A., Grosse-Wilde A., Janke M., Stahl H., Rieser E., Sprick M.R., Schirrmacher V. and Walczak H. (2003). TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle Disease Virus. J. Immun. 170 (4): 1814–1821.
Cella M., Salio M., Sakakibara Y., Langen H., Julkunen I. and Lanzavecchia A. (1999) Maturation, activation and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189: 821–829.
Bai L., Koopmann J., Fiola C., Fournier P. and Schirrmacher V. (2002). Dendritic cells pulsed with viral oncolysates potently stimulate autologous T cells from cancer patients. Int. J. Oncol. 21: 685–694.
Lodolce J.P., Burkett P.R., Boone D.L., Chien M., Ma A. (2001) T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J. Exp. Med. 194 (8):1187–1194.
Dubsky P., Saito H., Leogier M., Dantin C., Connolly J.E., Banchereau J., Palucka A.K. (2007) IL-15-induced human DC efficiently prime melanoma-specific naive CD8 + T cells to differentiate into CTL.Eur J Immunol. 37 (6): 1678–1690.
Von Hoegen P., Weber E. and Schirrmacher V. (1988) Modification of tumor cells by a low dose of Newcastle Disease Virus; augmentation of the tumor-specific T cell response in the absence of an anti-viral response. Eur. J. Immunology 18: 1159–1166.
Schild H.J., von Hoegen P. and Schirrmacher V. (1988). Modification of tumor cells by a low dose of Newcastle Disease Virus: II. Augmented tumor specific T cell response as a result of CD4 + and CD8 + immune T cell cooperation. Cancer Immunol. Immunother, 28: 22–28.
Von Hoegen P., Heicappell R., Griesbach A., Altevogt P. and Schirrmacher V. (1988). Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. III. Postoperative activation of tumor-specific CTLP from mice with metastases requires stimulation with the specific antigen plus additional signals. Proc. 8th Sapporo Cancer Seminar Invasion & Metastasis 9: 117–133.
Ertel C., Millar N.S., Emmerson P.T., Schirrmacher V. and von Hoegen P. (1993). Viral hemagglutinin augments peptide specific cytotoxic T-cell responses. Eur. J. Immunol. 23: 2592–2596.
Termeer C.C., Schirrmacher V., Bröcker E.B. and Becker J.C. (2000) Newcastle-Disease-Virus infection induces a B7–1/ B7–2 independent T-cell-costimulatory activity in human melanoma cells. Cancer Gene Ther. 7 (2): 316–323.
Haas C., Ertel C., Gerhards R. and Schirrmacher V. (1998) Introduction of adhesive and costimulatory immune functions into tumor cells by infection with Newcastle Disease Virus. Int. J. Oncol. 13: 1105–1115.
Washburn B. and Schirrmacher V. (2002) Human tumor cell infection by Newcastle Disease Virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int. J. Oncol. 21 (1): 85–93.
Takeda K., Kaisho T. and Akira S. (2003) Toll-like receptors. Annu Rev Immunol. 21: 335–76.
Thompson AJ and Locarnini SA. (2007) Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol. Cell. Biol. 85(6):435–445.
Kato H., Sato S., Yoneyama M., Yamamoto M., Uematsu S., Matsui K., Tsujimura T., Takeda K., Fujita T., Takeuchi O. and Akira S. (2005) Cell type-specific involvement of RIG-I in antiviral response. Immunity 23 (1): 19–28.
Melchjorsen J., Jensen S.B., Malmgaard L., Rasmussen S.B., Weber F., Bowie A.G., Matikainen S., Paludan S.R. (2005) Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J. Virol. 79 (20): 12944–12951.
Magyarics Z., Rajnavölgyi E. (2005) Professional type I interferon-producing cells—a unique subpopulation of dendritic cells. Acta Microbiol Immunol Hung. 52 (3–4): 443–462.
Kawai T. and Akira S. (2005). Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17: 338–344.
Schulz O., Diebold S.S., Chen M., Näslund T.I., Nolte M.A., Alexopoulou L., Azuma Y.T., Flavell R.A., Liljeström P., Reis E. and Sousa C. (2005) Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433 (7028): 887–892.
Lindenmann J. (1974) Viruses as immunological adjuvants in cancer. Biochim Biophys Acta 355 (1): 49–75.
Kyburz D., Aichele P., Speiser D.E., Hengartner H., Zinkernagel R.M., Pircher H. (1993) T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 23: 1956–1962.
Sato K., Hida S., Takayanagi H., Yokochi T., Kayagaki N., Takeda K., Yagita H., Okumura K., Tanaka N., Taniguchi T. and Ogasawara K. (2001) Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol 31: 3138.
Kumar-Sinha C., Varambally S., Sreekumar A. and Chinnaiyan A.M. (2002) Molecular cross-talk between the TRAIL and interferon signalling pathways. J. Biol. Chem. 277: 575–585.
Washburn B., Weigand M.A., Grosse-Wilde A., Janke M., Stahl H., Rieser E., Sprick M.R., Schirrmacher V. and Walczak H. (2003) TRAIL mediates tumoricidal activity of human monocytes stimulated by Newcastle Disease Virus. J. Immunol. 170: 1814–1821.
Le Bon A., Schiavoni G., D'Agostino G., Gresser I., Belardelli F. and Tough D.F. (2001) Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14 (4): 461–470.
Matzinger P. (1994) Tolerance, danger, and the extended family. Ann. Rev. Immunol. 12: 991–1045.
Matzinger P. (2002) The danger model: a renewed sense of self. Science 296: 301–305.
Forden C. (2004) Do T lymphocytes correlate danger signals to antigen? Med Hypotheses. 62 (6): 898–906.
Goodbourn, L. Didcock and Randall R.E. (2000) Interferons: cell signa, immune modulation, antiviral response and virus countermeasures, J. Gen. Virol. 81: 2341–2364.
Hengel U.H., Koszinowski U.H. and Conzelmann K.K. (2005) Viruses know it all new insights into IFN networks, Trends Immunol. 26: 396–401.
Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Biyagishi M, Taira K., Akira S. and Fujita T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5: 730–737.
Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R.A., Diamond M.S. and Colonna M. (2006) Essential role of MDA-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA. 103 (22): 8459–8464.
Hornung V., Ellegast J., Kim S., Brzózka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M., Endres S. and Hartmann G. (2006) 5'′-Triphosphate RNA is the ligand for RIG-I. Science. 314 (5801): 994–997.
Bowie A.G. and Fitzgerald K.A. (2007) RIG-I: tri-ing to discriminate between self and non-self RNA. Trends Immunol. 28 (4): 147–150.
Servant M.J., Tenoever B. and Lin R. (2002) Overlapping and distinct mechanisms regulating IRF-3 and IRF-7 function. J Interferon Cytokine Res. 22 (1): 49–58.
Levy D.E., Marié I., Smith E. and Prakash A. (2002) Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22 (1): 87–93.
Von Hoegen P., Zawatzky R. and Schirrmacher V. (1990) Modification of tumor cells by a low dose of Newcastle Disease Virus: III. Potentiation of tumor specific cytolytic T cell activity via induction of interferon alfa, beta. Cellular Immunology 126: 80–90.
Zeng J., Fournier P. and Schirrmacher V. (2002) Induction of interferon and tumor necrosis factor-related apoptosis-inducing blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle Disease Virus. Virology, 297: 19–30.
Rogge L., Barberis-Maino L., Biffi M., Passini N., Presky D.H., Gubler Sinigaglia E. (1997) Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185: 825.
Zeng, J., Fournier, P., Schirrmacher, V (2002) Stimulation of human natural interferon- response via paramyxo-virus hemagglutinin lectin-cell interaction. J. Mol. Med. 80: 443–451.
Fournier P., Zeng J., Von der Lieth C.W., Washburn B., Ahlert T. and Schirrmacher V. (2004) Importance of serine 200 for functional activities of the hemagglutinin-neuraminidase protein of Newcastle Disease Virus. Int. J. Oncol. 24: 623–634.
LeBon A. and Tough D.F. (2002) Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14: 432–426.
Tough D.F. (2004) Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma. 45 (2): 257–264.
Gallucci S. and Matzinger P. (2001) Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13: 114.
Bian H., Wilden H., Fournier P., Peeters B. and Schirrmacher V. (2006) In vivo efficacy of systemic tumor targeting of a viral RNA vector with oncolytic properties using a bispecific adapter protein. Int. J. Oncol. 29: 1359–1369.
Schirrmacher V. and Fournier P. (2006) Newcastle Disease Virus: a promising vector for viral therapy of cancer. In: Viral Therapy of Cancer, (Harrington KJ, Pandha HS and Vile RG eds) Wiley, New York (in press).
Lorence R.M., Roberts M.S., O'Neil J.D., Groene W.S., Miller J.A., Mueller S.N. and Bamat M.K. (2007) Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr. Cancer Drug Targets. 7 (2): 157–167.
Freeman A.I., Zakay-Rones Z., Gomori J.M., Linetsky E., Rasooly L., Greenbaum E., Rozenman-Yair S., Panet A., Libson E., Irving C.S., Galun E. and Siegal T. (2006) Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 13 (1): 221–228.
Pecora A.L., Rizvi N., Cohen G.I., Meropol N.J., Sterman D., Marshall J.L., Goldberg S., Gorss P., O'Neil J.D., Groene W.S., Roberts M.S., Rabin H., Bamat M.K. and Lorence R.M. (2002). Phase I trial of intravenous administration of PV 701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 20 (9): 2251–2266.
Hotte S.J., Lorence R.M., Hirte H.W., Polawski S.R., Bamat M.K., O'Neil J.D., Roberts M.S., Groene W.S. and Major P.P. (2007) An optimized clinical regimen for the oncolytic virus PV701. Clin. Cancer Res. 13 (3): 977–985.
Laurie S.A., Bell J.C., Atkins H.L., Roach J., Bamat M.K., O'Neil J.D., Roberts M.S., Groene W.S. and Lorence R.M. (2006) A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin. Cancer Res. 12 (8): 2555–2562.
Czegledi A., Wehmann E. and Lomniczi B. (2003). On the origins and relationships of Newcastle disease virus vaccine strains Hertfordshire and Mukteswar, and virulent strain Herts'33. Avian Pathol. 32: 271–276.
Csatary L.K., Moss R.W., Beuth J., Töröcsik B., Szeberenyi J. and Bakacs T. (1999) Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H). Anticancer Res. 19 (1B): 635–638.
Csatary L.K. and Bakács T. (1999) Use of Newcastle disease virus vaccine (MTH-68/H) in a patient with high-grade glioblastoma. JAMA 281 (17): 1588–1589.
Csatary L.K., Csatary C., Gosztonyi G. and Bodey B. (2006). Promising MTH-68/H Oncolytic Newcastle Disease Virus therapy in human high grade gliomas. Chapter IV In: Focus on Brain Cancer Research (Andrew V. Yang, ed.), Nova Science Publishers New York, pp. 69–82.
Csatary L.K., Eckhardt S., Bukosza I., Czegledi F., Fenyvesi C., Gergely P., Bodey B. and Csatary C.M. (1993) Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 17 (6): 619–627.
Position of the Scientific council on the antitumor studies conducted in Hungary on the Newcastle Disease Virus. (1998) Orv. Hetil. 139: 2903–2905.
Nelson N.J. (1999) Scientific interest in Newcastle Disease Virus is reviving. J. Natl. Cancer Inst. 91: 1708–1710.
Miller L.T. and Yates V.J. (1971) Reactions of human sera to avian adenoviruses and Newcastle disease virus. Avian Dis. 15(4): 781–788.
Charan S., Mahajan V.M. and Agarwal L.P. (1981) Newcastle disease virus antibodies in human sera. Indian J. Med. Res. 73: 303–307.
Van Pel A., Van der Bruggen P., Coulie P.G., Brichard. V.G., Lethe B., Van den Eynde. B., Uyttenhove C., Renauld. J.C. and Boon T. (1995) Genes coding for tumor antigens recognized by cytolytic T lymphocytes. Immunol. Rev. 145: 229–250.
Baxevanis C.N. and Papamichail M. (1994) Characterization of the anti-tumor immune response in human cancers and strategies for immunotherapy. Crit. Rev. Oncol. Hematol. 16: 157–179.
Schirrmacher V., Ahlert T., Pröbstle T., Steiner H.H., Herold-Mende C., Gerhards R., Hagmüller E. and Steiner H.H. (1998) Immunization with virus-modified tumor cells. Semin. Oncol. 25 (6): 677–696.
Steiner H.H., Bonsanto M.M., Beckhove P., Brysch M., Schuele-Freyer R., Geletneky K., Kremer P., Golamrheza R., Bauer H., Kunze S., Schirrmacher V. and Herold-Mende C.(2004)Anti-tumor vaccination of patients with glioblastoma multiforme: a pilot study to assess: Feasibility, safety and clinical benefit. J. Clin. Oncology 22 (21): 4272–4281
Gilboa E. (1999) The makings of a tumor rejection antigen. Immunity 11: 263–270
Coulie P.G., Lehman F., Lethé B., Herman J., Lurquin C., Andrawiss M. and Boon T.(1995)A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc. Natl. Acad. Sci. USA 92: 7976–7980
Lupetti R., Pisarra P., Verrecchia A., Farina C., Nicolini G., Anichini A., Bordignon C., Sensi M., Parmiani G. and Traversari C. (1998) Translation of a retained intron in tyrosinase-related protein (TRP) 2 mRNA generates a new cytotoxic T lymphocytes (CTL)-defined and shared human melanoma antigen not expressed in normal cells of the melanocytic lineage. J. Exp. Med. 188: 1005–1010.
Schirrmacher V. and Heicappell R. (1987). Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. II: establishment of specific systemic anti tumor immunity. Clin. Exp. Metastasis 5: 147–156.
Plaksin D., Porgador A., Vadai E., Feldman M., Schirrmacher V. and Eisenbach L. (1994) Effective anti-metastatic melanoma vaccination with tumor cells transfected with MHC genes and/or infected with Newcastle disease virus (NDV). Int. J. Cancer 59 (6): 796–801.
Shoham J., Hirsch R., Zakay-Rones Z., Osband M.E. and Brennert H.J. (1990) Augmentation of tumor cell immunogenicity by viruses—an approach to specific immunotherapy of cancer. Nat Immun Cell Growth Regul. 9 (3): 165–172.
Key M.E. and Hanna M.G. Jr. (1981) Mechanism of action of BCG-tumor cell vaccines in the generation of systemic tumor immunity. II. Influence of the local inflammatory response on immune reactivity. J. Natl. Cancer Inst. 67 (4): 863–869.
Schirrmacher V. (2005) Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol. Immunother. 54: 587–598.
Schirrmacher V., Haas C., Bonifer R., Ahlert T., Gerhards R. and Ertel C. (1999) Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle Disease Virus. Gene Ther. 6: 63–73.
Schirrmacher V., Feuerer M., Fournier P., Ahlert T., Umansky V. and Beckhove P. (2003) T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 9 (12): 526–534.
Schirrmacher V. and von Hoegen P. (1993) Importance of tumor cell membrane integrity and viability for CTL activation by cancer vaccines. Vaccine Res. 2: 183–196
Ahlert T., Sauerbrei W., Bastert G., Ruhland S., Bartik B., Simiantonaki N., Schumacher J., Häcker B., Schumacher M. and Schirrmacher V. (1997) Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J Clin Oncol. 15 (4): 1354–1366.
Schirrmacher V. (2005) Anti-tumor immune memory and its activation for control of residual tumor cells and improvement of patient survival. In: Virus Therapy of Human Cancers (Sinkovics, J., Horvath, J., eds), Marcel Decker, New York.
Lehner B., Schlag P., Liebrich W. and Schirrmacher V. (1990) Postoperative active specific immunization in curatively resected colorectal cancer patients with virus-modified autologous tumor cell vaccine. Cancer Immunol. Immuntherap. 32: 173–178.
Schlag P., Manasterski M., Gerneth Th., Hohenberger P., Dueck M., Herfarth Ch., Liebrich W. and Schirrmacher V. (1992). Active specific Immunotherapy with NDV modified autologous tumor cells following liver metastases resection in colorectal cancer: First evaluation of clinical response of a Phase II trial. Cancer Immunol. Immunother. 35: 325–330.
Bohle W., Schlag P., Liebrich W., Hohenberger P., Manasterski M., Möller P., and Schirrmacher V. (1990). Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumour cell vaccine: first clinical results with tumour cell vaccines modified with live but avirulent Newcastle Disease Virus. Cancer 66: 1517–1523.
Liebrich W., Schlag P., Manasterski M., Lehner B., Stöhr M. and Möller P., Schirrmacher V. (1991). In vitro and clinical characterization of a Newcastle Disease virus-modified autologous tumor cell vaccine for treatment of colorectal cancer patients. Europ. Journal Cancer 27: 703–710.
Ockert D., Schirrmacher V., Beck N., Stoelben E., Ahlert T., Flechtenmacher J., Hagmüller E., Nagel M. and Saeger H.D. (1996). Newcastle Disease Virus infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin. Cancer Res. 2: 21–28.
Pomer S., Schirrmacher V., Thiele R., Löhrke H. and Staehler G. (1995). Tumor response and 4 year survival data of patients with advanced renal cell carcinoma treated with autologous tumor vaccine and subcutaneous r-IL-2 and IFN-Alpha 2b. Int. J. Oncol. 6: 947–954.
Ahlert T., Sauerbrei W., Bastert G., Ruhland S., Bartik B., Simiantonaki N., Schumacher, J., Häcker B., Schumacher M., and Schirrmacher V. (1997) Tumor cell number and viability as quality and efficacy parameters of autologous virus modified cancer vaccines. J. Clin. Oncol. 15: 1354–1366.
Karcher J., Dyckhoff G., Beckhove P., Reisser C., Brysch M., Ziouta Y., Helmke B., Weidauer H., Schirrmacher V. and Herold-Mende C. (2004). Anti-tumor vaccination with HNSCC with autologous virus-modified tumor cells. Cancer Res. 64 (21): 8057–8061.
Haas C., Lulei M., Fournier P., Arnold A. and Schirrmacher V. (2005) T-cell triggering by CD3- and CD28-binding molecules linked to a human virus-modified tumor cell vaccine. Vaccine 23: 2439–2453.
Haas C., Lulei M., Fournier P., Arnold A. and Schirrmacher V. (2005) A tumor vaccine containing anti-CD3 and anti-CD28 bispecific antibodies triggers strong and durable anti-tumor activity in human lymphocytes. Int. J. Cancer 118 (3): 658–667.
Bian H., Fournier P., Moormann R., Peeters B. and Schirrmacher V. (2005) Selective gene transfer in vitro to tumor cells via recombinant Newcastle Disease Virus. Cancer Gene Ther. 12: 295–303.
Bian H., Fournier P., Moormann R., Peeters B. and Schirrmacher V. (2005) Selective gene transfer to tumor cells by recombinant Newcastle Disease Virus via a bispecific fusion protein. Int. J. Oncol. 26: 431–439.
Bian H., Fournier P., Peeters B. and Schirrmacher V. (2005) Tumor-targeted gene transfer in vivo via recombinant Newcastle Disease Virus modified by a bispecific fusion protein. Int. J. Oncol. 27: 377–384.
Conzelmann K.K. (1998) Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Ann. Rev. Genet. 32: 123–162.
García-Sastre A. (1998) Negative-strand RNA viruses: applications to biotechnology. Trends Biotechnol. 16: 230–235.
Nagai Y. and Kato A. (1999) Paramyxovirus reverse genetics is coming of age. Microbiol. Immunol. 43: 613–624.
Roberts A. and Rose J.K. (1998) Recovery of negative-strand RNA viruses from plasmid DNAs: a positive approach revitalizes a negative field. Virology 247: 1–6.
Peeters B.P.H., de Leeuw O.S., Koch G. and Gielkens A.L.J. (1999) Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 73: 5001–5009.
Römer-Oberdörfer A., Mundt E., Mebatsion T., Buchholz U. and Mettenleiter T.C. (1999) Generation of recombinant lentogenic Newcastle disease virus from cDNA. J. Gen. Virol. 80: 2987–2995.
Mebatsion T., Verstegen S., De Vaan L.T., Römer-Oberdörfer A. and Schrier C.C. (2001). A recombinant Newcastle Disease Virus with low-level V protein expression is immunogenic and lacks pathogenicity for chicken embryos. J. Virol. 75: 420–428.
Vigil A., Park M.S., Martinez O., Chua M.A., Xiao S., Cros J.F., Martínez-Sobrido L., Woo S.L. and García-Sastre A. (2007) Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 67 (17): 8285–8292.
Zhao H. and Peeters B.P. (2003) Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J. Gen. Virol. 84 (Pt 4): 781–788.
Huang Z., Krishnamurthy S., Panda A. and Samal S.K. (2001) High-level expression of a foreign gene from the most 3'′-proximal locus of a recombinant Newcastle disease virus. J. Gen. Virol. 82 (Pt 7): 1729–1736.
Nakaya T., Cros J., Park M.S., Nakaya Y., Zheng H., Sagrera A., Villar E., García-Sastre A. and Palese P. (2001) Recombinant Newcastle Disease Virus as a vaccine vector. J. Virol. 75: 11868–11873.
Bureyev A., Huang Z., Yang L., Elankumaran S., St. Claire M., Murphy B.R., Samal S.K. and Collins P.L. (2005). Recombinant Newcastle Disease Virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J. Virol. 79: 13275–13284.
Ge J., Deng G., Wen Z., Guobing T., Wang Y., Shi J., Wang X., Li Y., Hu S., Jiang Y., Yang C., Yu K., Bu Z. and Chen H. (2007). Newcastle Disease Virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 81 (1): 150–158.
Sivasamugham L.A., Cardosa M.J., Tan W.S. and Yusoff K. (2006) Recombinant Newcastle Disease virus capsids displaying enterovirus 71 VP1 fragment induce a strong immune response in rabbits. J. Med. Virol. 78 (8): 1096–1104.
Veits J., Wiesner D., Fuchs W., Hoffmann B., Granzow H., Starick E., Mundt E., Schirrmeier H., Mebatsion T., Mettenleiter T.C. and Römer-Oberdörfer A. (2006) Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc. Natl. Acad. Sci. USA 103 (21): 8197–8202.
Huang Z., Elankumaran S., Yunus A.S., Samal S.K. (2004) A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 78 (18): 10054–10063.
Alexander D.J. (1988) Newcastle disease virus - an avian paramyxovirus In: Newcastle Disease (Alexander D.J. ed), Kluwer, Boston, pp. 11–22.
Acknowledgment
The work done by our group around Professor Schirrmacher would have not been possible without the dedicated help and contributions of many basic scientists and clinicians. Their names can be obtained from the list of the quoted references.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Humana Press, a part of Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Schirrmacher, V., Fournier, P. (2009). Newcastle Disease Virus: A Promising Vector for Viral Therapy, Immune Therapy, and Gene Therapy of Cancer. In: Walther, W., Stein, U. (eds) Gene Therapy of Cancer. Methods in Molecular Biology™, vol 542. Humana Press. https://doi.org/10.1007/978-1-59745-561-9_30
Download citation
DOI: https://doi.org/10.1007/978-1-59745-561-9_30
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-934115-85-5
Online ISBN: 978-1-59745-561-9
eBook Packages: Springer Protocols