Overview of SERPING1 Variations Identified in Hungarian Patients With Hereditary Angioedema
- 1Department of Internal Medicine and Hematology, Semmelweis University, Budapest, Hungary
- 2Hungarian Angioedema Center of Reference and Excellence, Semmelweis University, Budapest, Hungary
- 3Second Department of Pediatrics, Semmelweis University, Budapest, Hungary
Background: Hereditary angioedema (HAE) due to C1-inhibitor (C1-INH) deficiency (C1-INH-HAE) is a rare autosomal dominant disorder, characterized by recurrent, unpredictable edematous symptoms involving subcutaneous, and/or submucosal tissue. C1-INH-HAE may be caused by more than 700 different mutations in the gene encoding C1-INH (SERPING1) that may lead to decreased protein synthesis or to functional deficiency.
Methods: Concentrations of C1-INH, C4, C1q, and anti-C1-INH antibodies, as well as functional C1-INH activity were determined in subjects suffering from edematous symptoms and admitted to the Hungarian Angioedema Center of Reference and Excellence. In those patients, who were diagnosed with C1-INH-HAE based on the complement measurements, SERPING1 was screened by bidirectional sequencing following PCR amplification and multiplex ligation-dependent probe amplification. For detecting large deletions, long-range PCRs covering the entire SERPING1 gene by targeting 2–7 kb long regions were applied.
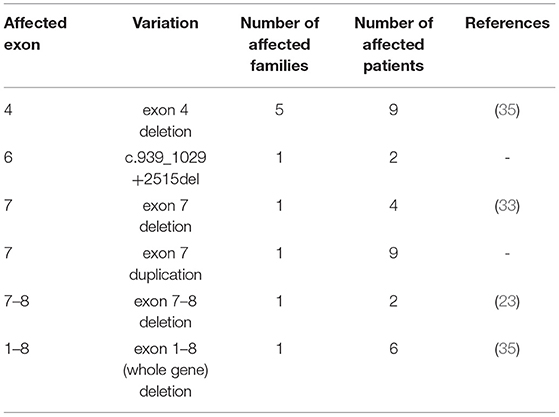
Results: Altogether 197 individuals with C1-INH deficiency belonging to 68 families were identified. By applying Sanger sequencing or copy number determination of SERPING1 exons, 48 different mutations were detected in 66/68 families: 5 large and 15 small insertions/deletions/delins, 16 missense, 6 nonsense, and 6 intronic splice site mutations. Two novel variations (p.Tyr199Ser [c.596A>C] and the duplication of exon 7) were shown to cosegregate with deficient C1-inhibitor level and activity, while two other variations were detected in single patients (c.797_800delinsCTTGGAGCTCAAGAACTTGGAGCT and c.812dup). A series of long PCRs was applied in the remaining 2 families without an identified mutation and a new, 2606 bp long deletion including the last 91 bp of exon 6 (c.939_1029+2515del) was identified in all affected members of one pedigree. In the remaining one family, a deep intronic SERPING1 variation (c.1029+384A>G) was detected by a targeted next-generation sequencing panel as reported previously.
Conclusions: Sequencing and copy number determination of SERPING1 exons uncover most pathogenic variants in C1-INH-HAE patients, and further methods are worth to be applied in cases with unrevealed genetic background. Since knowledge of the genetic background may support the establishment of the correct and early diagnosis of C1-INH-HAE, identification of causative mutations and reporting data supporting the interpretation on the pathogenicity of these variants is of utmost importance.
Introduction
Hereditary angioedema (HAE) due to C1-inhibitor (C1-INH) deficiency (C1-INH-HAE) is a rare autosomal dominant disorder, characterized by recurrent, unpredictable, nonpitting edematous symptoms involving the subcutaneous and/or submucosal tissues and showing intra- and interindividual variability. C1-INH-HAE episodes usually affect the extremities (arms, hands, feet, and legs), the face, lips, eyelids, bowels, and genitalia. In those rare cases when the edema evolves in the upper airways, the disease may lead to a potentially life-threatening condition within hours, without the proper treatment. Since the first pathogenic SERPING1 mutation was described in 1987 (1), the presence of C1-INH-HAE was explained by more than 700 different mutations (2–4) in the gene encoding C1-INH (SERPING1) that can either cause decreased protein synthesis (C1-INH-HAE type I) or functional deficiency (C1-INH-HAE type II). The mutations in the background of C1-INH-HAE type II are usually missense mutations affecting the reactive center loop of C1-INH encoded in exon 8. On the other hand, variations leading to the development of C1-INH-HAE type I are quite heterogeneous and are distributed over the exons and introns of the entire SERPING1 gene, in form of deletions or insertions of various sizes along with missense or nonsense substitutions, leading to the defect of C1-INH synthesis or secretion.
Molecular genetic testing is not considered as obligatory to confirm the diagnosis of C1-INH-HAE, though analyzing the segregation of novel SERPING1 variants in case of available family members is recommended to confirm the pathogenicity and penetrance (5). Considering the facts that (1) SERPING1 variants are predominantly associated with decreased functional C1-INH activity and that (2) C1-INH-HAE is inherited in an autosomal dominant pattern with high penetrance, novel SERPING1 mutations need only in a few cases to be functionally characterized in vitro, when considering their pathogenicity (6, 7).
Establishing the correct and early diagnosis of C1-INH-HAE (including the screening of all symptomatic and asymptomatic first-degree relatives of the diagnosed patients) is of high importance for the affected subjects' proper treatment, prognosis, and quality of life. Furthermore, previously asymptomatic family members may – in the future – suffer unpredictable symptoms that require specific treatment. Knowledge of a pedigree's causative SERPING1 mutation is also useful as this information may contribute to preimplantation and prenatal diagnosis or underpin diagnosis in cases with uncertain complement results that may occur in very early childhood (8).
Here, we explain our strategy on genetic work-up exploring the SERPING1 gene and provide an overview of SERPING1 mutations identified in Hungarian C1-INH-HAE patients over the past decades involving those published previously as well as new families with novel mutations.
Materials and Methods
In the Hungarian Angioedema Center of Reference and Excellence, 197 individuals (110 female, 87 male, mean age 42.8 years) belonging to 68 families were identified with C1-INH deficiency. The diagnosis of C1-INH-HAE was established according to the international consensus criteria (9), based on the following complement measurements: serum concentration of C1-INH was determined by radial immunodiffusion (10), C4 level by immunoturbidimetry (Beckman Coulter, Brea, CA, USA), levels of anti-C1-INH antibodies and C1q were measured by ELISA (11, 12), whereas the functional C1-INH activity was analyzed by using a commercial kit (Quidel, San Diego, CA, USA) in the serum samples of subjects suffering from edematous symptoms.
In those patients, who were diagnosed with C1-INH-HAE based on the complement measurements, and also in their available family members, genomic DNA was isolated from peripheral or umbilical cord blood samples by the salting-out method (13).
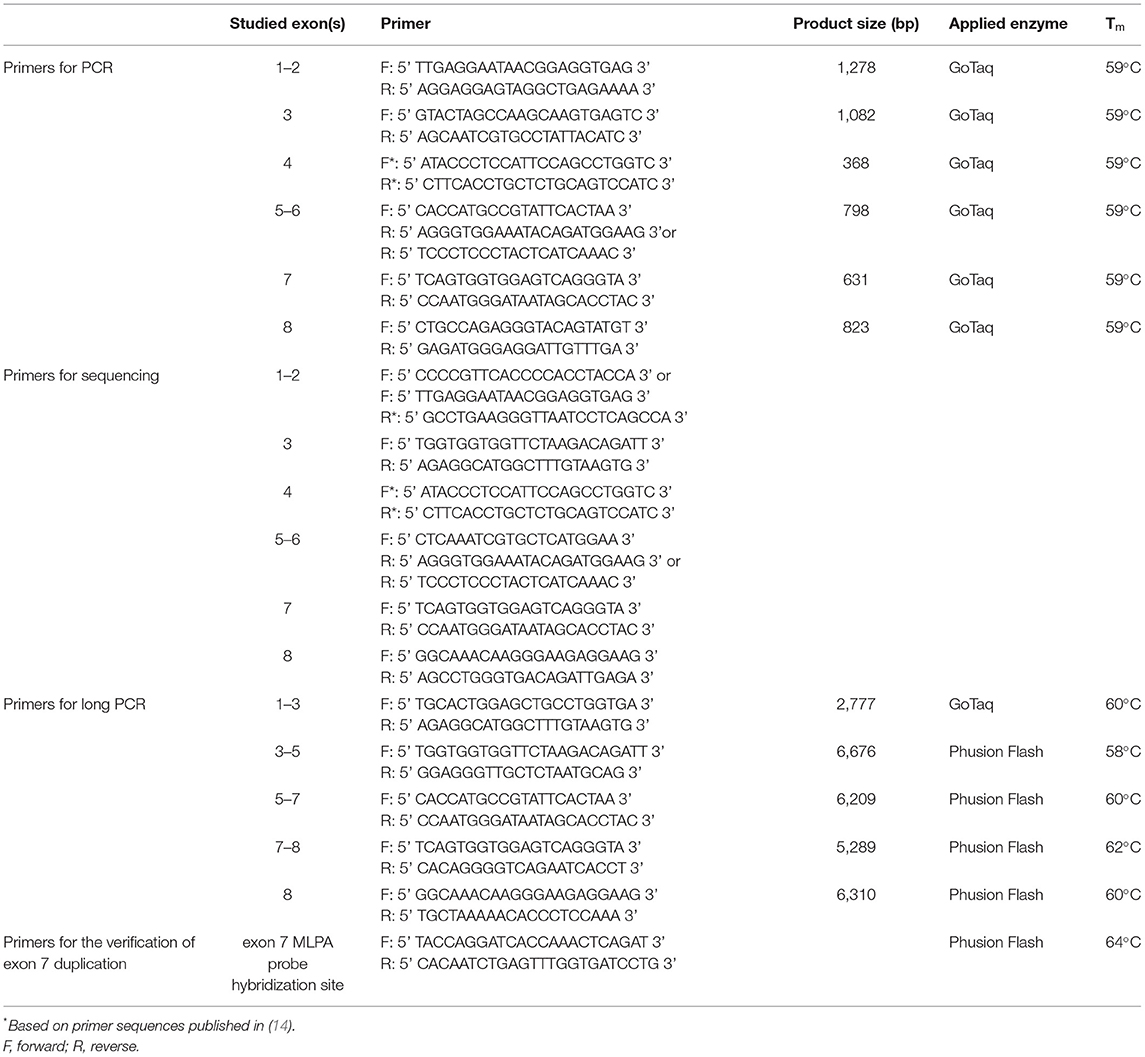
Bidirectional DNA sequencing following PCR amplification was applied to screen the whole coding region of the gene encoding C1-inhibitor (SERPING1; OMIM #606860). Amplification of genomic DNA was carried out in 35 cycles with GoTaq G2 DNA polymerase (Promega, Madison, WI, USA) according to the manufacturer's instructions (details of the reactions are available upon request) applying the primers listed in Table 1. Before sequencing, PCR products were purified with Exonuclease I and FastAP Thermosensitive Alkaline Phosphatase (Thermo Scientific, Waltham, MA, USA) and sequencing was performed using the BigDye Terminator v3.1 Cycles Sequencing Kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions with the sequencing primers specified in Table 1. After sodium acetate/ethanol purification, sequencing products were separated with an Applied Biosystems 3130xl Genetic Analyzer (Life Technologies, Carlsbad, CA, USA).
In order to detect large deletions or duplications in the SERPING1 gene, multiplex ligation-dependent probe amplification (MLPA) was performed applying the SALSA MLPA P243-A3 or P243-B1 SERPING1-F12 probemixes (MRC Holland, Amsterdam, The Netherlands). Data were analyzed using the Coffalyser.Net™ MLPA analysis software (MRC Holland, Amsterdam, The Netherlands) according to the manufacturers' instructions.
A series of long-range PCRs amplifying 2–7 kb sequences was performed with Promega GoTaq G2 DNA polymerase (Promega, Madison, WI, USA) or Phusion Flash PCR Master Mix (Thermo Scientific, Waltham, MA, USA) in cases of unresolved genetic background and their family members using the primers listed in Table 1.
The identified genetic variations were named according to the Human Genomic Variation Society (HGVS) recommendations (15) and for cDNA nucleotide numbering the reference sequence of SERPING1 (ID: NM_000062.3) was used. Interpretation of sequence variants was based on the criteria established by the American College of Medical Genetics and Genomics (ACMG) (16). The possible functional effect of an identified novel rare variation was assessed using in silico prediction tools, such as Sorting Intolerant From Tolerant (SIFT) (http://siftdna.org/www/Extended SIFT chrcoords submit.html)(17), PolyPhen (version 2) (http://genetics.bwh.harvard.edu/pph2/) (18), PROVEAN (http://provean.jcvi.org/genomesubmit.php) (19), CADD (https://cadd.gs.washington.edu/) (20), Mutation-Taster (http://mutationtaster.org) (21), or Human Splicing Finder (22) (version 3.1; http://www.umd.be/HSF3/~[21]).
Results
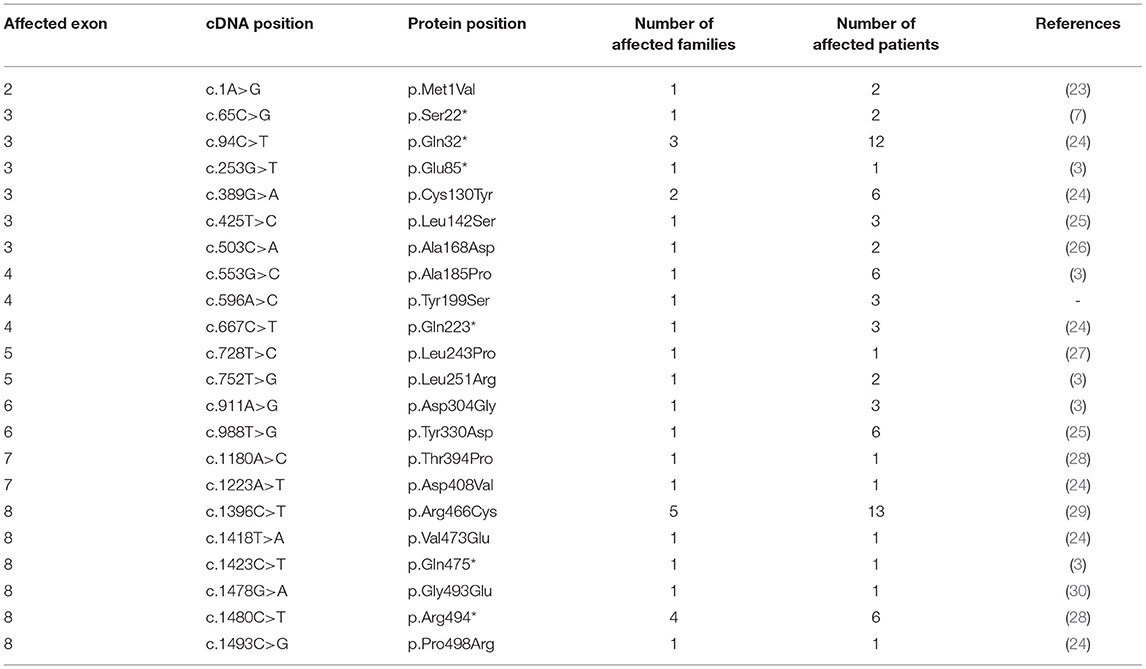
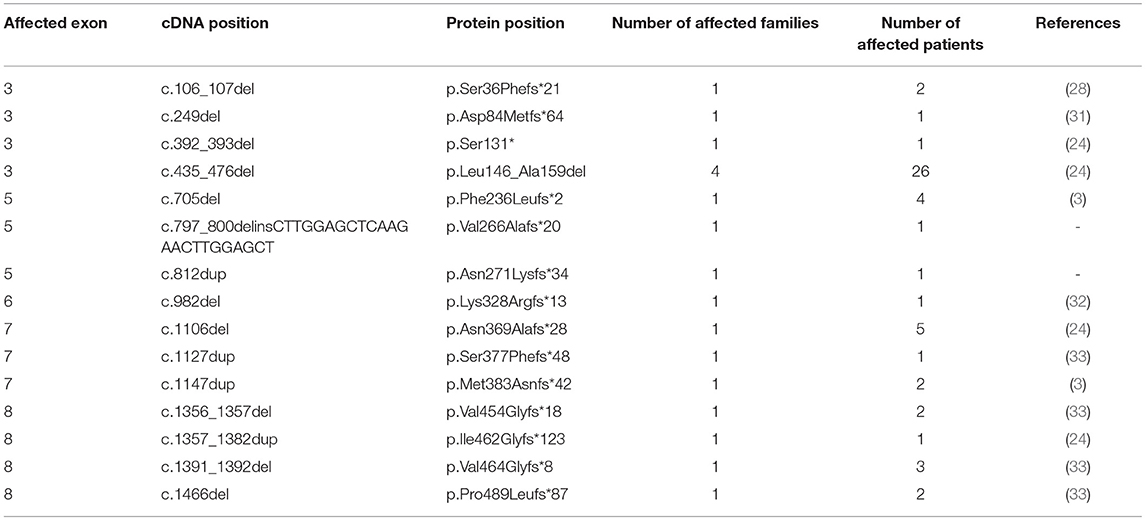
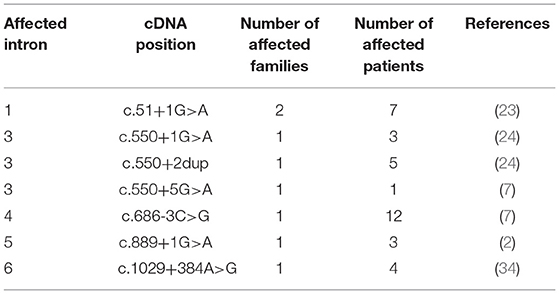
By applying Sanger sequencing to screen coding exons and exon–intron boundaries of the SERPING1 gene, 43 different mutations were identified in 57 of the studied 68 families (Figure 1). Alterations changing the coding region of SERPING1 included 10 small deletions, 4 small duplications, 1 delins, and 22 substitutions, the latter resulting in 16 missense and 6 nonsense variations, while 6 mutations influenced the intronic splice sites (Tables 2–5). All the cases (13 patients in 5 families) with C1-INH-HAE type II carried the p.Arg466Cys missense variation.
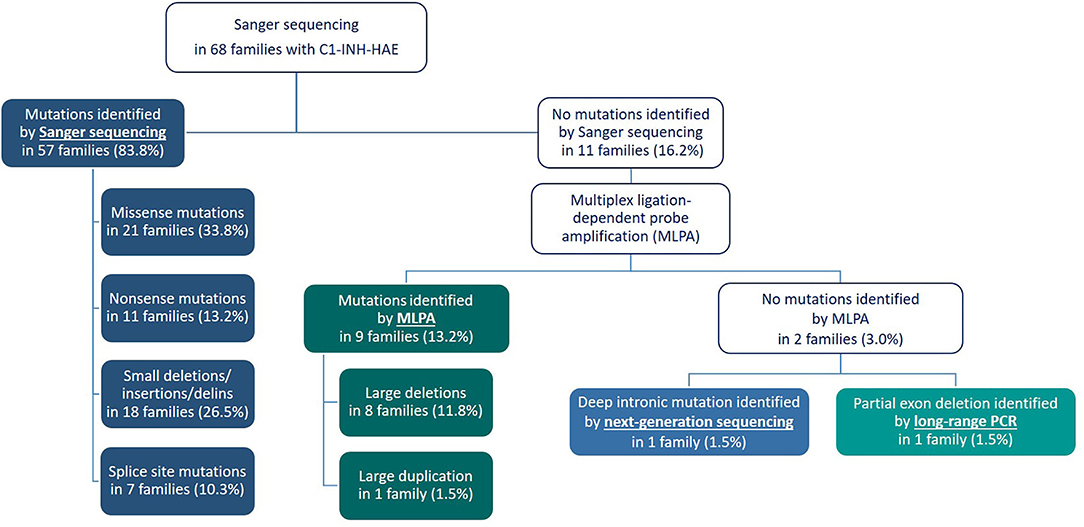
Figure 1. Flow chart illustrating the algorithm for molecular genetic testing in the Hungarian Angioedema Center of Reference and Excellence.
Among the detected substitutions, one novel (c.596A>C) was identified that causes a tyrosine to serine amino acid change at codon 199 (Tyr199Ser) located in the enzymatically active serpin domain. The potential effect of this missense variation was estimated by five different prediction programs, with 4 out of the 5 programs predicting the change to be disease causing and one to be neutral (SIFT: damaging [score: 0.049]; PROVEAN: deleterious [score: −5.03]; Mutation Taster: disease-causing [probability: 0.67]; CADD PHRED-like score: 23.1; Polyphen-2: benign [score: 0.250] [sensitivity: 0.91; specificity: 0.88] by the HumDiv model). Available family members were also tested for the carrier state of the mutation, and the affected sister and their mother were found to be heterozygous for this substitution and both of them showed impaired complement results (low C4 with deficient C1-INH function and level).
A novel single base duplication (c.812dup) was identified in a patient with low C1-INH level and activity. By the insertion of an adenine in exon 5, this mutation causes frameshift and introduces a premature stop codon (p.Asn271Lysfs*34) in the mRNA. Two of the applied prediction tools were suitable for estimating the potential effect of a duplication; both indicated a deleterious effect for this variation with high probability (Mutation Taster: disease-causing [probability: 1]; CADD PHRED-like score: 26.3). No other rare variation was detected in the patient, who had no available family members.
A complex variation with the deletion of 4 nucleotides and the insertion of 24 bp (c.797_800delinsCTTGGAGCTCAAGAACTTGGAGCT) was also identified that causes a frameshift and premature termination of protein synthesis at the amino acid position 266 (p.Val266Alafs*). The patient who carried this mutation did not have any other rare variation in the SERPING1 gene and her complement measurements showed deficient C1-inhibitor level as well as below-normal C4 level. Segregation could not be verified in this case as no family members were available for testing.
In those patients in whom no sequence alteration was found with Sanger sequencing or the pathogenicity of the identified variation was not undoubtedly supported, copy number of SERPING1 exons was studied by MLPA (Figure 1). A part of our patient group was analyzed previously with Southern blot technique combined with relative quantification of SERPING1 exons using real-time PCR and SybrGreen detection (24), and the MLPA provided concordant result in each case. Five different deletions involving one or more SERPING1 exons were identified all of which were reported previously in C1-INH-HAE patients (Table 5). MLPA results showed three copies of exon 7 in case of each available affected members of a large family (Figure 2) and as this mutation was not reported previously, patients from this family were further investigated in order to verify the duplication of exon 7 with an independent method. Since MLPA indicated an extra copy of the site recognized by the exon 7 probe, forward and reverse PCR primers (listed in Table 1) were designed for this region to amplify the sequence between the duplicated exon 7 probe binding sites. Applying this PCR, an app. 4 kb product was generated in samples of each affected individual of the corresponding family but not from healthy controls (data not shown). Complement measurements showed deficient C1-INH activity and level in the available members with edematous symptoms, while normal complement results were obtained from the analyzed healthy relatives (Figure 2).
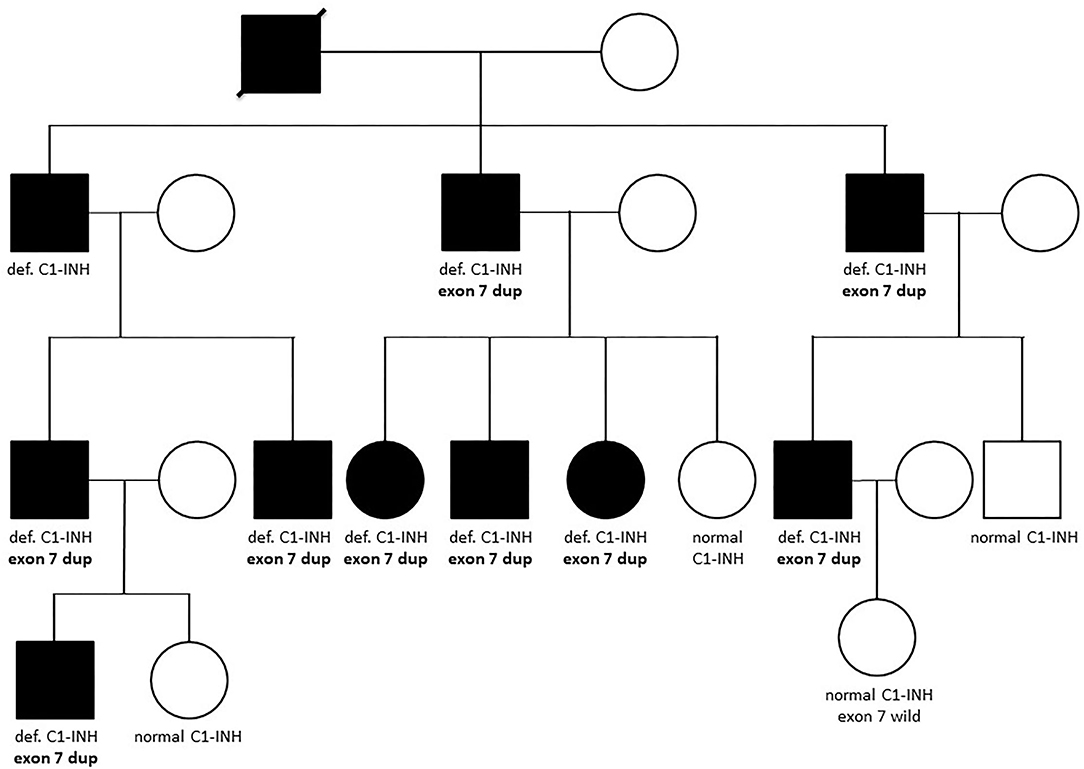
Figure 2. Family tree of a patient with the novel SERPING1 exon 7 duplication. Affected members of the family are depicted with dark symbols, while all available results of complement and genetic measurements are denoted below the symbols. Def. or normal C1-INH means deficient or normal activity and level of C1-inhibitor, respectively. “Exon 7 dup” refers to the heterozygous carrier state of the duplication of SERPING1 exon 7, while individuals denoted as “exon 7 wild” does not carry this variation.
In the case of two families, MLPA showed two copies for each studied exon and a normal sequence was retrieved from Sanger sequencing of exons and exon–intron boundaries in the patients. However, these methods have limitations and may overlook certain mutations, such as deletions that does not involve the recognition site of any of the MLPA probes or mutations that affect one or both of the PCR primer binding sites. To overcome this problem, a series of long-range PCRs was applied that cover the whole SERPING1 gene with 2–7 kb long products. As Figure 3 shows an extra, smaller band was observed in case of the long PCR amplifying the region of exon 5–7 and sequencing of this product revealed that the patient carries a 2606 bp long deletion including the last 91 bp of exon 6 and 2515 bp of intron 6 (c.939_1029+2515del). This particular long-range PCR was applied to analyze the available family members of the patient showing that his affected father also carried this deletion but PCR performed from the samples of his symptom-free mother and aunt (the father's sister) revealed only wild-type products. In agreement with this, deficient C1-inhibitor activity and level were detected only in the index patient and his father.
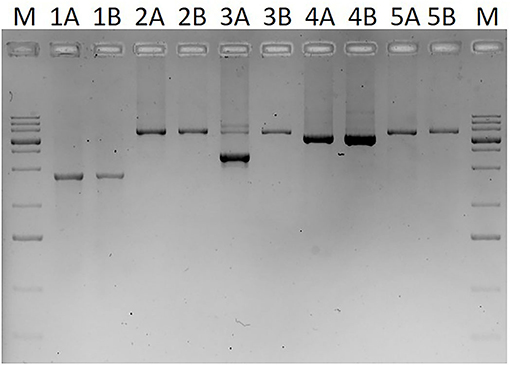
Figure 3. Image of agarose gel (1%) electrophoresis of long-range PCR products covering the whole SERPING1 gene. M: GeneRuler 1 kb plus DNA ladder (Thermo Scientific, Waltham, MA, USA). PCR products in lines involve the following exons: lines 1: exons 1–3 (from the binding site of the MLPA probe specific for exon 1), lines 2: exons 3–5, lines 3: exons 5–7, lines 4: exons 7–8, lines 5: exon 8 (plus an extra 1375 bp downstream from the 3'-end of exon 8). Samples marked with A (1A−5A) are derived from a C1-INH-HAE patient, samples with B (1B−5B) are from a healthy control.
In the remaining one family, a deep intronic SERPING1 variation (c.1029+384A>G) was detected by a targeted next-generation sequencing (NGS) panel as reported previously (34). In order to verify this variant with Sanger sequencing and to screen for its carrier state in new patients, a new reverse primer was designed for the previously used forward one and since then applied in the diagnostic work-up (Table 1).
Besides the mutation identified as pathogenic, two rare variations were detected in two families, a sense variant (p.Leu251=) in the two affected members of a family with exon 4 deletion and a rare missense polymorphism (p.Arg366His) in a patient without available family members, who also carried the p.Glu85* nonsense mutation (3).
As a probable causative variation was detected in each of the studied Hungarian families, the diagnosis based on complement measurements was verified by genetic testing in each of the patients. Knowledge of the SERPING1 mutation allowed the verification of C1-inhibitor deficiency in 24, at the time of the analysis yet symptom-free individuals. These subjects included 11 children under the age of 1 year, when complement levels (especially C4) may be lower than the normal adult levels in healthy children as well (36). Among them, 5 were newborns, in whom the analyzed DNA was isolated from the umbilical cord. Moreover, exclusion of C1-INH-HAE was achieved from the umbilical cord DNA of 4 offspring including two cases with ambiguous complement results (below normal C4 and C1-INH level with normal C1-INH function).
Discussion
Based on the current guidelines, molecular genetic testing is not obligatory as a first-line diagnostic approach in case of patients with angioedema, as most of the cases can be diagnosed based on the clinical picture, complement laboratory findings (functional and antigenic levels of C1-inhibitor, concentrations of C4, C1q, and autoantibodies against C1-inhibitor) as well as family history (if available) (9). However, it is still of high importance to report the identified variations that (1) have a clear damaging effect on protein function/level, or that (2) are segregating with the disease or that (3) are confirmed to be pathogenic by functional studies, as their publication or inclusion in disease-specific databases may help further investigators to decide about their pathogenicity.
Here, we report the mutational spectrum of a large cohort of Hungarian C1-INH-HAE patients diagnosed in the Hungarian Angioedema Center of Reference and Excellence, the national center caring all Hungarian C1-INH-HAE patients. This is an update of the whole Hungarian cohort diagnosed in the past almost 50 years including families with previously published genetic background (3, 24, 32–34, 37, 38), but extended with further family members and also newly diagnosed patients with the identified SERPING1 mutations. By applying conventional molecular genetic methods such as Sanger sequencing and MLPA, a pathogenic SERPING1 variation could be explored in almost all families: C1-INH deficiency was caused by a missense or nonsense mutation in 47.1% (32/68), by a small deletion, insertion, or delins in 26.5% (18/68), by a large deletion or duplication in 13.2% (9/68) or by an intronic variation in 10.3% (7/68) of the analyzed pedigrees.
Among missense variations one novel was identified, an adenine to cytosine substitution in exon 4, causing a tyrosine to serine change at codon 199 of the C1-inhibitor protein. This novel substitution was not found in international databases studying large populations with different ethnicity (gnomAD: https://gnomad.broadinstitute.org/; 1,000 Genomes project: https://www.internationalgenome.org/; NHLBI GO Exome Sequencing Project: https://evs.gs.washington.edu/EVS/). Applying five prediction tools to assess its pathogenicity, ambiguous results were retrieved, however, with a dominance of damaging predictions (4 out of 5). The pathogenic role of this p.Tyr199Ser variation is supported by several factors, one of which is that the two carrier children in the family have recurrent angioedema attacks and their complement parameters are typical of C1-INH-HAE. Coinheritance of deficient C1-inhibitor level and activity with the mutation was also observed as the mother with insufficient level and activity of C1-inhibitor is a mutation carrier as well. However, segregation of the mutation with symptoms was not clearly confirmed as she only once had an attack of suspicious abdominal pain, but never experienced subcutaneous edematous episodes. The pathogenicity of the identified p.Tyr199Ser variation located in the helix-rich region of the serpin domain is also underpinned by a previous study that described a pathogenic tyrosine–asparagine change at this position (p.Tyr199Asn), indicating the importance of the tyrosine at codon 199 (7).
Another novel single nucleotide variation, the insertion of an adenine (c.812dup) was detected in a patient without available family members who had decreased level of C1-INH and C4 as well as deficient functional activity of C1-INH. The pathogenicity of this mutation is clear, as by the duplication of one base it shifts the reading frame and results in the generation of a premature termination codon in the formed mRNA.
In our C1-INH-HAE cohort, five gene rearrangements were identified by MLPA: four previously published large deletions involving 1 to 8 exons and a duplication of exon 7 which was not reported previously. The presence of this novel copy number variation was verified by long-range PCR in each affected individual of the corresponding family; moreover, this large pedigree allowed the detection of clear cosegregation of the mutation with C1-INH-HAE symptoms and deficient complement levels. Besides, amplification of an app. 4 kb product by applying forward and reverse primers specific for the MLPA exon 7 probe hybridization site supported the close (within gene) location of the extra copy of this site.
After performing the analysis of SERPING1 gene with Sanger sequencing and MLPA, two families (2.9% of the pedigrees analyzed by our group) still remained in which no mutation could be identified. This is in agreement with previous studies (3, 27) and a meta-analysis of Ponard et al. who described that no SERPING1 gene alterations could be identified using Sanger and MLPA methods in 24 families fulfilling the clinical and laboratory criteria of C1-INH-HAE (24/379; 6.3%) (2). Patients in these two families were further analyzed by a series of long PCRs that was developed in order to detect medium-sized alterations that involve only a small part of an exon and not affect hybridization sites of the MLPA probes. These kinds of mutations are not detected by MLPA and if the binding site of the forward or reverse PCR primer is also deleted, it won't be detected by Sanger sequencing either as only the wild-type allele will be amplified from the other chromosome. Long-range PCR is a widely accepted method to investigate large gene rearrangements, and was applied by several groups in the genetic diagnosis of HAE as well (3, 27, 39). These PCRs were usually designed to amplify extra-large products (sometimes more than 10–15 kb) in order to capture large deletions, or even deletion of the whole gene; however, capacity of these methods to detect deletions affecting only a few hundred or thousand nucleotides is limited. By applying the MLPA technique, we were able to detect large rearrangements; therefore, in this study, we rather focused on smaller deletions and applied PCR primers to amplify 2–7 kb products. With this method, a partial deletion was detected involving the last 91 bp of exon 6 and 2,515 bp of intron 6 in the two symptomatic cases but not in two healthy members of the corresponding family. Functional relevance of this mutation is obvious either by causing the deletion of 91 bp from the coding sequence resulting in reading frameshift or by abolishing the exon–intron boundary impairing correct splicing. Moreover, the clear cosegregation of this mutation with deficient C1-INH function and level as well as edematous symptoms also confirms its pathogenicity.
In one family, the standard approaches failed to uncover the disease-causing SERPING1 alteration; therefore, a NGS platform targeting the entire SERPING1 gene was performed that resulted in the identification of a deep intronic variation (c.1029+384A>G) as published in details previously (34). Intronic regions were initially considered to be mostly nonfunctional and variations found deep within the introns (i.e., in a distance of more than 100 base pairs away from the exon–intron boundaries) were disregarded as pathogenic alterations having any functional consequences. However, with the advent of whole-genome sequencing a few novel deep intronic variants were identified in SERPING1 that showed a clear association with the symptoms of C1-INH-HAE based on recent studies, supporting the functional importance of the intronic sequences (6, 40). As Hujová's study nicely presented the SERPING1 c.1029+384A>G mutation results in de novo donor splice site creation and subsequent pseudoexon inclusion (6).
In each affected family of our C1-INH-HAE cohort of almost 200 subjects, we were able to detect a variant that was classified as “pathogenic” or “potentially pathogenic” based on the criteria of ACMG (16). Identifying the disease-causing SERPING1 variation could be highly useful in those cases where the clinical diagnosis of C1-INH-HAE is highly suspected but the complement test results are inconclusive. This occurs frequently before or upon the first presentation of HAE-like symptoms (such as unexplained gastrointestinal symptoms) in children with a positive family history of C1-INH-HAE, where the functional and antigenic C1-INH levels are not reliable parameters of C1-INH-HAE before the first year of age, as their reference ranges are significantly lower compared to the adult reference ranges (8, 9, 41). Knowledge of the causative mutation allowed us to perform genetic analysis immediately after birth from umbilical cord samples of newborns with a parent suffering from C1-INH-HAE. In five cases, sequencing or copy number analysis of the corresponding exon(s) indicated inheritance of the SERPING1 mutation, while it excluded C1-INH-HAE in four newborns (including two cases with uncertain complement results). Besides, determination of the mutation carrier state verified the diagnosis in further 6 children under the first year of age when complement measurements may be inconclusive.
Considering the routinely used molecular genetic approaches such as bidirectional sequencing of SERPING1 exons and the exon/intron boundaries along with the copy number determination of SERPING1 exons, these are able to explore most of the disease-causing variations in C1-INH-HAE patients. However, in those families where complement laboratory studies suggest the diagnosis of C1-INH-HAE but no SERPING1 mutation can be identified by the conventional methods used in a diagnostic laboratory, or in case the detected missense SERPING1 variation is supposed to be benign according to previous publications, databases or in silico prediction tools, further unique techniques should be applied in order to explore the pathogenic alteration in the background of the disease. Discovering SERPING1 variations in C1-INH-HAE patients and reporting data that contribute to the correct interpretation of the pathogenicity of these variations are of utmost importance since knowledge of the genetic background may support the establishment of the correct and early diagnosis of C1-INH-HAE promoting the patient's proper treatment, prognosis, and quality of life.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Research Council of the Ministry of Human Capacities in Hungary (approval number: 55381-1/ 2015/EKU) and the institutional review board of the Semmelweis University, Budapest. Written informed consent to patients, or participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
ES, DC, and ÁS: performed genetic analyses, participated in writing, and technical editing of the manuscript. NA: collected patient's data. LV: managed complement measurements. HF: provided and cared for study patients. The final version of the article was read and approved by all the contributors. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by NKFI 124557.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We express our special thanks to Anastasios E. Germenis, Matthaios Speletas, Maria Zamanakou, Sofia Vatsiou, Faidra Parsopoulou, Fotis Psarros, Maria Kompoti, Gedeon Loules, Attila Tordai, András Bors, and Lajos Kalmár for contributing to the molecular genetic analysis of the previously published mutations. We also thank Judit Bali, Beáta Takács, Kata Marossy, Zsuzsanna Szendrei, Andrásné Dóczy, and Lászlóné Kertész for their expert technical assistance.
Abbreviations
ACMG, American College of Medical Genetics and Genomics; CADD, Combined Annotation Dependent Depletion; C1-INH, C1-inhibitor; C1-INH-HAE, Hereditary angioedema due to C1-inhibitor deficiency; HAE, Hereditary angioedema; MLPA, Multiplex ligation-dependent probe amplification; PCR, polymerase chain reaction; SIFT, Sorting Intolerant From Tolerant.
References
1. Stoppa-Lyonnet D, Tosi M, Laurent J, Sobel A, Lagrue G, Meo T. Altered C1 inhibitor genes in type I hereditary angioedema. N Engl J Med. (1987) 317:1–6. doi: 10.1056/NEJM198707023170101
2. Ponard D, Gaboriaud C, Charignon D, Ghannam A, Wagenaar-Bos IGA, Roem D, et al. SERPING1 mutation update: mutation spectrum and C1 Inhibitor phenotypes. Hum Mutat. (2020) 41:38–57. doi: 10.1002/humu.23917
3. Speletas M, Szilagyi A, Psarros F, Moldovan D, Magerl M, Kompoti M, et al. Hereditary angioedema: molecular and clinical differences among European populations. J Allergy Clin Immunol. (2015) 135:570–3. doi: 10.1016/j.jaci.2014.08.007
4. Loli-Ausejo D, Lopez-Lera A, Drouet C, Lluncor M, Phillips-Angles E, Pedrosa M, et al. In Search of an Association Between Genotype and Phenotype in Hereditary Angioedema due to C1-INH Deficiency. Clin Rev Allergy Immunol. (2021) 61:1–14. doi: 10.1007/s12016-021-08834-9
5. Germenis AE, Margaglione M, Pesquero JB, Farkas H, Cichon S, Csuka D, et al. International Consensus on the Use of Genetics in the Management of Hereditary Angioedema. J Allergy Clin Immunol Pract. (2020) 8:901–11. doi: 10.1016/j.jaip.2019.10.004
6. Hujova P, Soucek P, Grodecka L, Grombirikova H, Ravcukova B, Kuklinek P, et al. Deep Intronic Mutation in SERPING1 Caused Hereditary Angioedema Through Pseudoexon Activation. J Clin Immunol. (2020) 40:435–46. doi: 10.1007/s10875-020-00753-2
7. Roche O, Blanch A, Duponchel C, Fontan G, Tosi M, Lopez-Trascasa M. Hereditary angioedema: the mutation spectrum of SERPING1/C1NH in a large Spanish cohort. Hum Mutat. (2005) 26:135–44. doi: 10.1002/humu.20197
8. Nielsen EW, Johansen HT, Holt J, Mollnes TE. C1 inhibitor and diagnosis of hereditary angioedema in newborns. Pediatr Res. (1994) 35:184–7. doi: 10.1203/00006450-199402000-00012
9. Maurer M, Magerl M, Ansotegui I, Aygoren-Pursun E, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy. (2018) 73:1575–96. doi: 10.1111/all.13384
10. Kelemen Z, Moldovan D, Mihaly E, Visy B, Szeplaki G, Csuka D, et al. Baseline level of functional C1-inhibitor correlates with disease severity scores in hereditary angioedema. Clin Immunol. (2010) 134:354–8. doi: 10.1016/j.clim.2009.11.002
11. Delamarche C, Berger F, Pouplard A, Emile J. An ELISA technique for the measurement of C1q in cerebrospinal fluid. J Immunol Methods. (1988) 114:101–6. doi: 10.1016/0022-1759(88)90160-3
12. Varga L, Fust G, Csuka D, Farkas H. Treatment with C1-inhibitor concentrate does not induce IgM type anti-C1 inhibitor antibodies in patients with hereditary angioedema. Mol Immunol. (2011) 48:572–6. doi: 10.1016/j.molimm.2010.10.012
13. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. (1988) 16:1215. doi: 10.1093/nar/16.3.1215
14. Zuraw BL, Herschbach J. Detection of C1 inhibitor mutations in patients with hereditary angioedema. J Allergy Clin Immunol. (2000) 105:541–6. doi: 10.1067/mai.2000.104780
15. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat. (2016) 37:564–9. doi: 10.1002/humu.22981
16. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
17. Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC, et al. web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. (2012) 40:W452–7. doi: 10.1093/nar/gks539
18. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. (2010) 7:248–9. doi: 10.1038/nmeth0410-248
19. Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE. (2012) 7:e46688. doi: 10.1371/journal.pone.0046688
20. Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. (2019) 47:D886–94. doi: 10.1093/nar/gky1016
21. Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. (2010) 7:575–6. doi: 10.1038/nmeth0810-575
22. Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. (2009) 37:e67. doi: 10.1093/nar/gkp215
23. Gosswein T, Kocot A, Emmert G, Kreuz W, Martinez-Saguer I, Aygoren-Pursun E, et al. Mutational spectrum of the C1INH (SERPING1) gene in patients with hereditary angioedema. Cytogenet Genome Res. (2008) 121:181–8. doi: 10.1159/000138883
24. Kalmar L, Bors A, Farkas H, Vas S, Fandl B, Varga L, et al. Mutation screening of the C1 inhibitor gene among Hungarian patients with hereditary angioedema. Hum Mutat. (2003) 22:498. doi: 10.1002/humu.9202
25. Loules G, Zamanakou M, Parsopoulou F, Vatsiou S, Psarros F, Csuka D, et al. Targeted next-generation sequencing for the molecular diagnosis of hereditary angioedema due to C1-inhibitor deficiency. Gene. (2018) 667:76–82. doi: 10.1016/j.gene.2018.05.029
26. Suffritti C, Zanichelli A, Maggioni L, Bonanni E, Cugno M, Cicardi M. High-molecular-weight kininogen cleavage correlates with disease states in the bradykinin-mediated angioedema due to hereditary C1-inhibitor deficiency. Clin Exp Allergy. (2014) 44:1503–14. doi: 10.1111/cea.12293
27. Pappalardo E, Caccia S, Suffritti C, Tordai A, Zingale LC, Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol. (2008) 45:3536–44. doi: 10.1016/j.molimm.2008.05.007
28. Verpy E, Biasotto M, Brai M, Misiano G, Meo T, Tosi M. Exhaustive mutation scanning by fluorescence-assisted mismatch analysis discloses new genotype-phenotype correlations in angiodema. Am J Hum Genet. (1996) 59:308–19.
29. Skriver K, Radziejewska E, Silbermann JA, Donaldson VH, Bock SC. CpG mutations in the reactive site of human C1 inhibitor. J Biol Chem. (1989) 264:3066–71. doi: 10.1016/S0021-9258(18)94031-7
30. Blanch A, Roche O, Lopez-Granados E, Fontan G, Lopez-Trascasa M. Detection of C1 inhibitor (SERPING1/C1NH) mutations in exon 8 in patients with hereditary angioedema: evidence for 10 novel mutations. Hum Mutat. (2002) 20:405–6. doi: 10.1002/humu.9073
31. Guryanova I, Suffritti C, Parolin D, Zanichelli A, Ishchanka N, Polyakova E, et al. Hereditary angioedema due to C1 inhibitor deficiency in Belarus: epidemiology, access to diagnosis and seven novel mutations in SERPING1 gene. Clin Mol Allergy. (2021) 19:3. doi: 10.1186/s12948-021-00141-0
32. Balla Z, Zsilinszky Z, Polai Z, Andrasi N, Kohalmi KV, Csuka D, et al. The Importance of Complement Testing in Acquired Angioedema Related to Angiotensin-Converting Enzyme Inhibitors. J Allergy Clin Immunol Pract. (2021) 9:947–55. doi: 10.1016/j.jaip.2020.08.052
33. Bors A, Csuka D, Varga L, Farkas H, Tordai A, Fust G, et al. Less severe clinical manifestations in patients with hereditary angioedema with missense C1INH gene mutations. J Allergy Clin Immunol. (2013) 131:1708–11. doi: 10.1016/j.jaci.2012.11.015
34. Germenis AE, Vatsiou S, Csuka D, Zamanakou M, Farkas H. Deep Intronic SERPING1 Gene Variants: Ending One Odyssey and Starting Another? J Clin Immunol. (2021) 41:248–50. doi: 10.1007/s10875-020-00887-3
35. Duponchel C, Di Rocco C, Cicardi M, Tosi M. Rapid detection by fluorescent multiplex PCR of exon deletions and duplications in the C1 inhibitor gene of hereditary angioedema patients. Hum Mutat. (2001) 17:61–70. doi: 10.1002/1098-1004(2001)17:1<61::AID-HUMU7>3.0.CO;2-9
36. Farkas H, Martinez-Saguer I, Bork K, Bowen T, Craig T, Frank M. A, et al. Zanichelli, and Hawk, International consensus on the diagnosis and management of pediatric patients with hereditary angioedema with C1 inhibitor deficiency. Allergy. (2017) 72:300–13. doi: 10.1111/all.13001
37. Kalmar L, Hegedus T, Farkas H, Nagy M, Tordai A. HAEdb: a novel interactive, locus-specific mutation database for the C1 inhibitor gene. Hum Mutat. (2005) 25:1–5. doi: 10.1002/humu.20112
38. Raffai I, Fust G, Koo E, Hajos M. Hereditary angioneurotic edema, analysis of 3 cases. Orv Hetil. (1972) 113:2471–4.
39. Lopez-Lera A, Garrido S, Roche O, Lopez-Trascasa M. SERPING1 mutations in 59 families with hereditary angioedema. Mol Immunol. (2011) 49:18–27. doi: 10.1016/j.molimm.2011.07.010
40. Vatsiou S, Zamanakou M, Loules G, Psarros F, Parsopoulou F, Csuka D, et al. A novel deep intronic SERPING1 variant as a cause of hereditary angioedema due to C1-inhibitor deficiency. Allergol Int. (2020) 69:443–9. doi: 10.1016/j.alit.2019.12.009
Keywords: C1-inhibitor, C1-INH-HAE, hereditary angioedema, long-range PCR, MLPA, mutation, sequencing, SERPING1
Citation: Szabó E, Csuka D, Andrási N, Varga L, Farkas H and Szilágyi Á (2022) Overview of SERPING1 Variations Identified in Hungarian Patients With Hereditary Angioedema. Front. Allergy 3:836465. doi: 10.3389/falgy.2022.836465
Received: 15 December 2021; Accepted: 07 February 2022;
Published: 17 March 2022.
Edited by:
Allen P. Kaplan, Medical University of South Carolina, United StatesReviewed by:
Milos Jesenak, Comenius University, SlovakiaSira Nanthapisal, Thammasat University, Thailand
Copyright © 2022 Szabó, Csuka, Andrási, Varga, Farkas and Szilágyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ágnes Szilágyi, szilagyi.agnes@med.semmelweis-univ.hu
 Edina Szabó
Edina Szabó Dorottya Csuka
Dorottya Csuka Noémi Andrási
Noémi Andrási Lilian Varga
Lilian Varga Henriette Farkas
Henriette Farkas Ágnes Szilágyi
Ágnes Szilágyi