- 1Neuropsychiatry Service, Hunter New England Mental Health, Newcastle, NSW, Australia
- 2Centre for Translational Neuroscience and Mental Health, University of Newcastle, Newcastle, NSW, Australia
Traumatic brain injury (TBI) is a common condition that is often complicated by neuropsychiatric sequelae that can have major impacts on function and quality of life. An alteration in the sense of smell is recognized as a relatively common complication of TBI; however in clinical practice, this complication may not be sought or adequately characterized. We conducted a systematic review of studies concerned with olfactory functioning following TBI. Our predetermined criteria led to the identification of 25 studies published in English, which we examined in detail. We have tabulated the data from these studies in eight separate tables, beginning with Table 1, which highlights each study’s key findings, and we provide a summary/synthesis of the findings in the accompanying results and discussion sections. Despite widely differing methodologies, the studies attest to a high frequency of post-TBI olfactory dysfunction and indicate that its presence can serve as a potential marker of additional structural or functional morbidities.
Introduction
Traumatic brain injury (TBI) is a common, potentially preventable cause of mortality, and major morbidity. Neuropsychiatric sequelae, including cognitive, behavioral, and psychiatric symptoms and signs, may be present to varying degrees following a TBI according to the premorbid characteristics of the patient, its nature and severity, and the time elapsed since the injury. When specifically sought, olfactory functioning disturbances are common following TBI and, if present, can have a significant impact on quality of life (1). Reduced appreciation of food, drink, and other smell-laden sensual experiences; loss of employment, when this depends on an intact sense of smell; and increased danger from environmental hazards (e.g., volatile agents/gas, fires, spoiled food) are among the most obvious potential consequences of post-TBI olfactory deficits.
The availability in recent years of standardized instruments for assessing olfaction has enabled researchers to investigate with greater precision and rigor the associations between TBI (and related phenomena) and altered olfactory functioning (2, 3). However, despite the functional relevance of olfactory impairments following TBI, systematic objective quantitative testing of olfaction is not, at least in our own clinical experience, routinely undertaken. In light of this and because the substantial and growing literature suggests that olfactory impairment is relatively common and clinically important, we thought that a systematic review would be timely.
In the course of this review, issues of high clinical relevance are raised that, as far as we are aware, have not recently been systematically examined within the same work including: what relationship exists between TBI severity and the risk for post-TBI olfactory impairment? How commonly does olfactory impairment arise following a TBI? What are the structural and functional correlates of post-TBI olfactory impairment? What is the prognosis for post-TBI olfactory impairment? What is the impact of post-TBI olfactory impairment on quality of life?
Materials and Methods
The review was conducted in two stages. In stage 1, articles were retrieved via online database searching. The online databases of PsycINFO, MEDLINE, EMBASE, Scopus, and COCHRANE were searched. Keywords and combinations of these words were used to search the databases comprehensively: diffuse axonal injury, brain hemorrhage, intracranial hemorrhage, brain edema, penetrating head injuries, olfaction disorders, olfactory perception, olfactory bulb, olfactory pathways, olfactory mucosa, olfactory receptor neurons, TBI, olfaction, olfactory, head injury, sense of smell, brain injury, smell, odor/odor, hyposmia, anosmia, brain damage, brain concussion, cerebral concussion, concussion, and craniocerebral trauma. Articles were limited to those that were published in English-language journals from 1980 to August 2013 and to studies in humans.
During stage 2, the titles and abstracts of articles were reviewed to assess eligibility for inclusion in this review. Articles were regarded as relevant and warranting inclusion in the review if they were human studies, using validated olfactory testing methods in distinct TBI populations. Where there was uncertainty about whether a study should be included based on the review of the title and abstract, the full article was retrieved (see Figure 1 for article exclusion results).
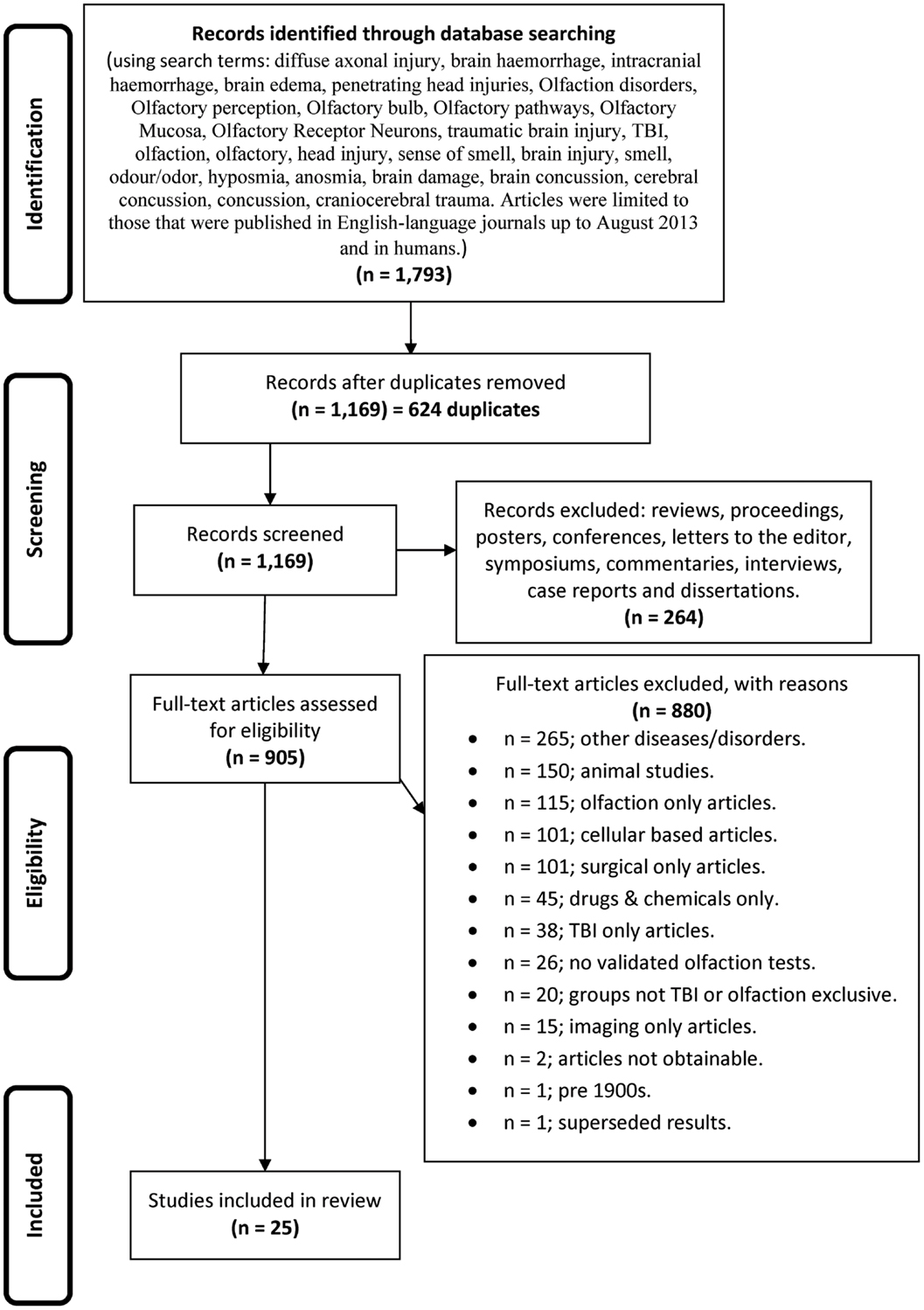
Figure 1. The flow diagram depicting the search strategy, process, and exclusionary criteria by which the studies were selected for inclusion in this systematic review.
Data Extraction
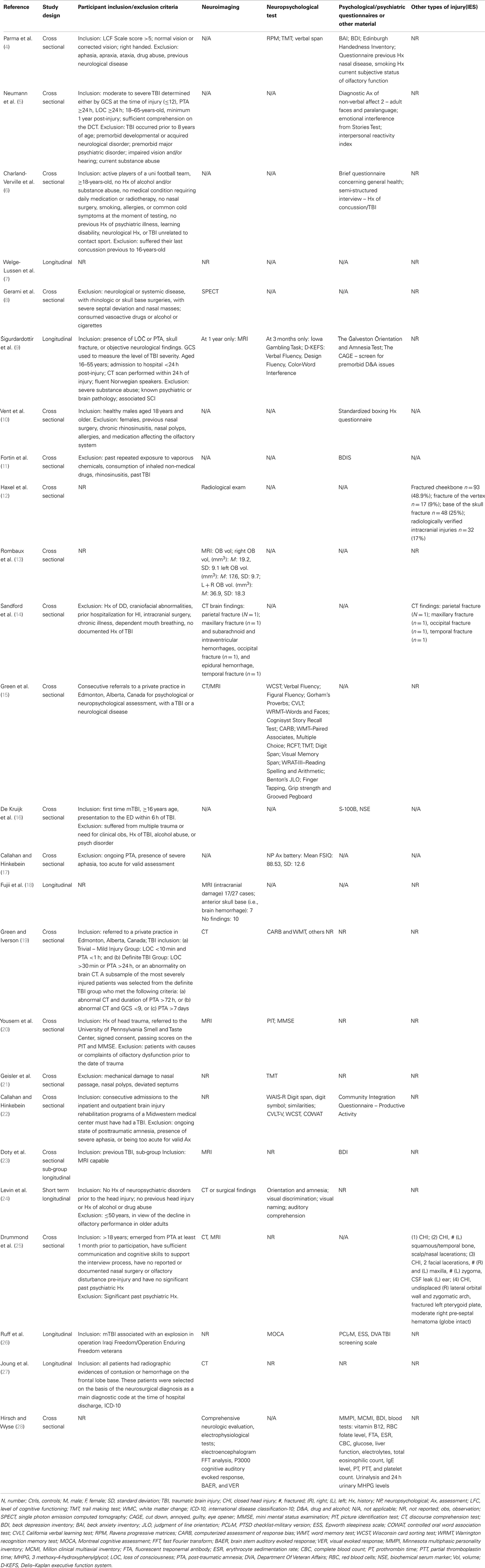
Initially, one reviewer extracted data from the identified studies, including (1) participant demographics (TBI and control subjects), (2) characteristics of participants (TBI severity, duration of loss of consciousness (LOC), mechanism of injury), (3) olfactory testing paradigms (technique and data extraction), (4) time, elapsed since injury, (5) results of the study, and (6) study findings.
Results
Our initial searches, based on the search strategies described in Figure 1, generated 1,793 hits. After duplicates were removed, we were left with 1,169 records, which were screened leading to removal of a further 264 records, leaving 905 full text articles that were further assessed for eligibility. As indicated in Figure 1, a further 880 were culled for the reasons listed.
Scope, Definitions, and Methodology of the Studies
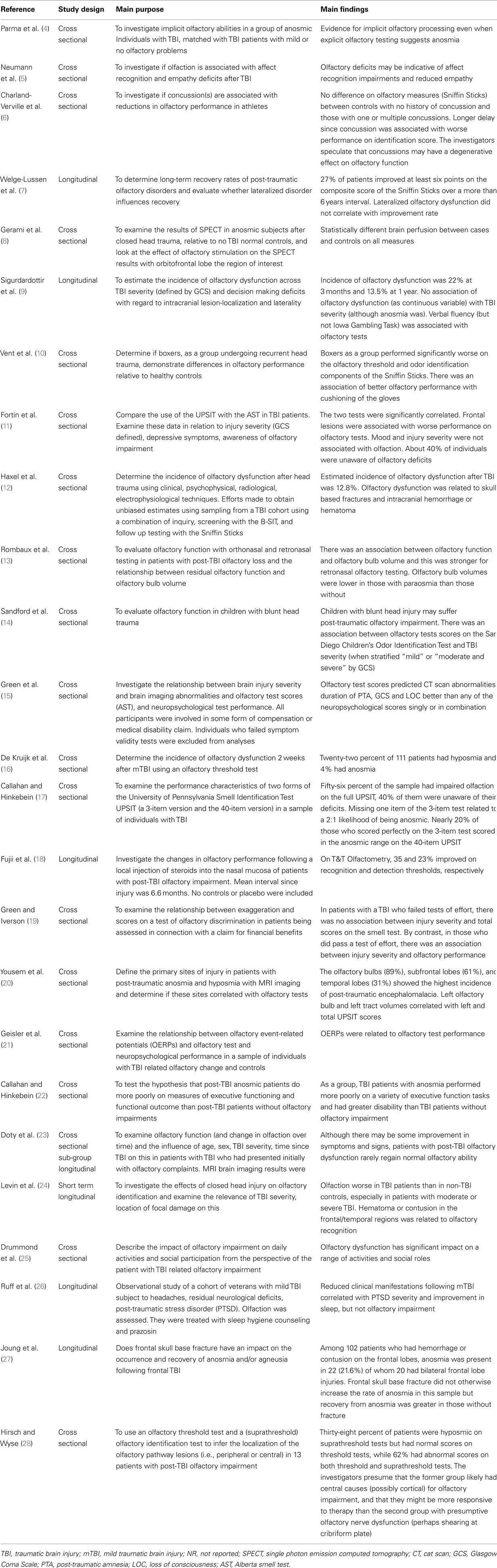
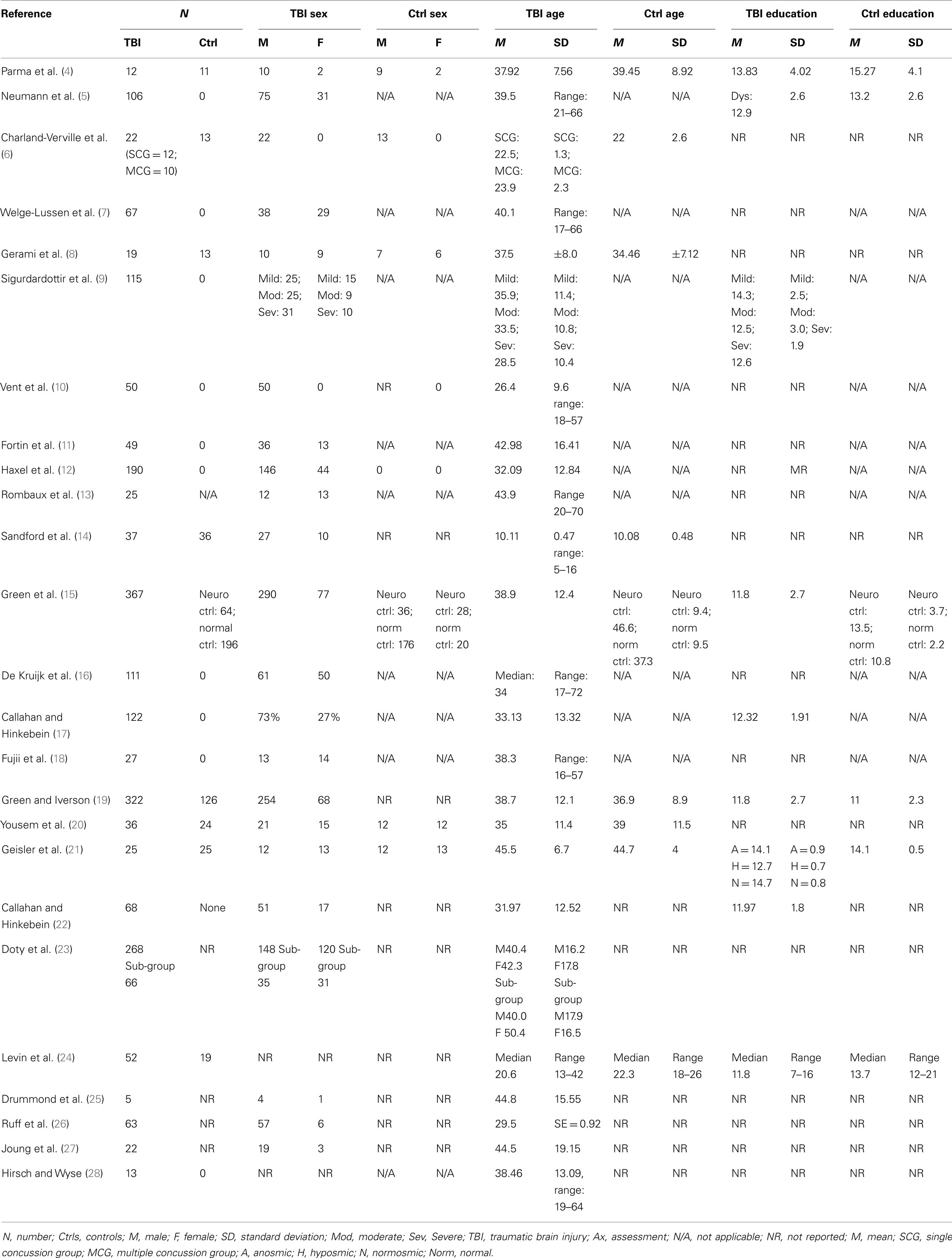
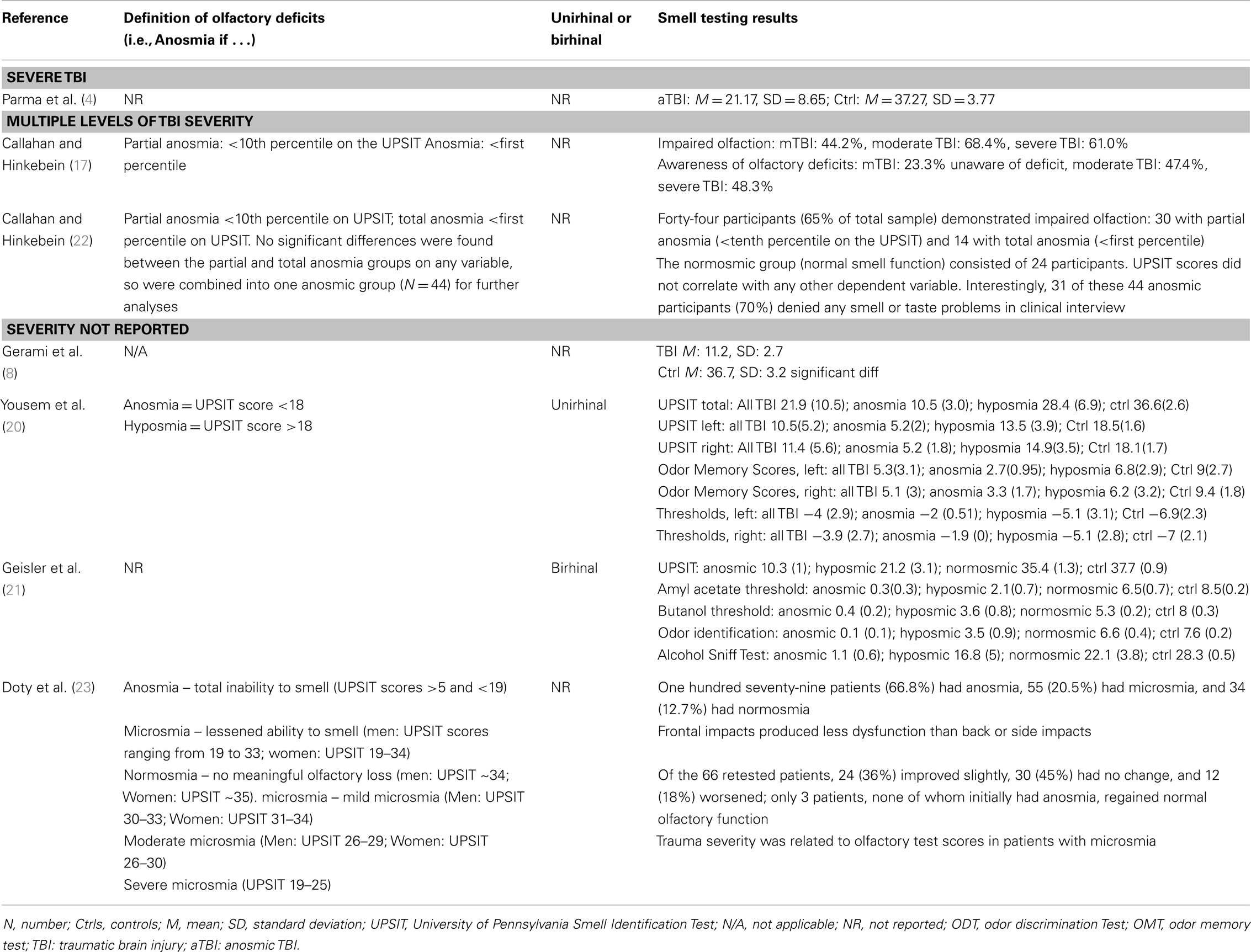
Table 1 provides a brief overview of the main goals and findings of each of the 25 studies that we examined in detail (4–28) and the subsequent Tables 2–8 provide further detail regarding aspects of the methodology, sample characteristics, etc. The studies ranged broadly in terms of their principal focus (Table 1). Most of the studies included individuals who had sustained a TBI, regardless of the presence or otherwise of olfactory problems (5, 6, 9–12, 14–17, 19, 21–24, 26, 27) while in eight studies participants with TBI were selected for the presence of olfactory complaints or established olfactory impairment (4, 7, 8, 13, 18, 20, 25, 28). While most of the studies were cross sectional in design, several had an important longitudinal aspect (7, 9, 18, 26). The individual study sample sizes ranged from 5 individuals in the single qualitative study (25) to 367 (15). Only one of the studies was concerned with children with TBI specifically (14) (Table 2).
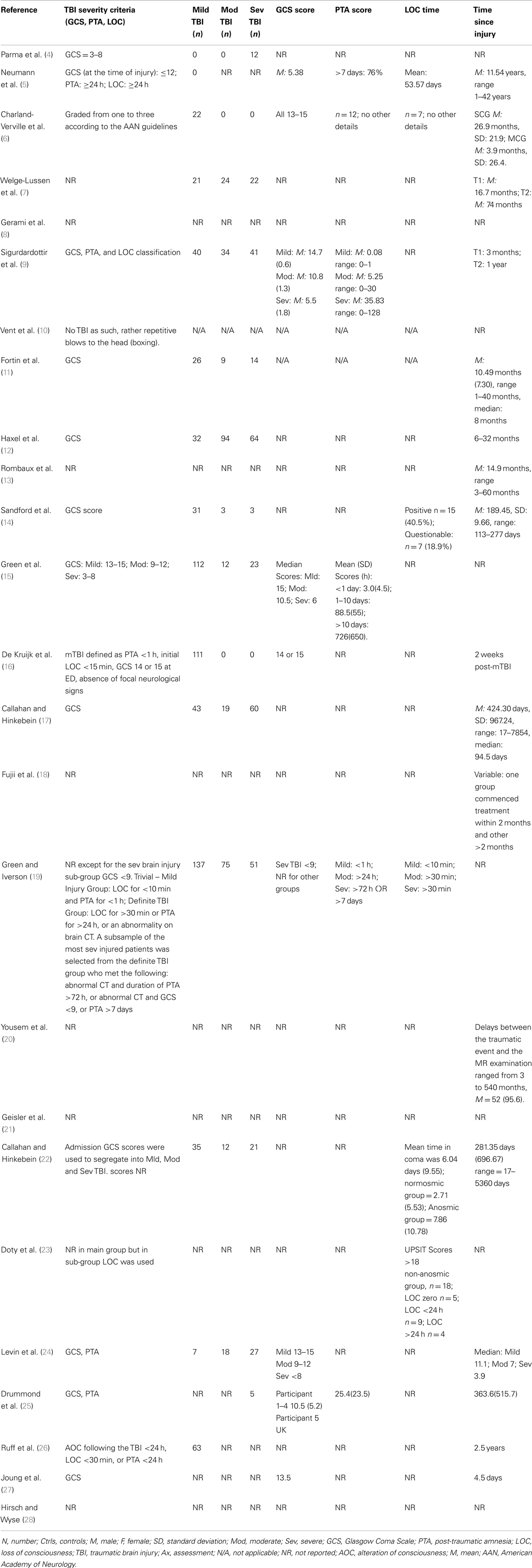
Traumatic brain injury severity was variously defined by the Glasgow Coma Score (GCS) (5, 6, 9, 15, 16, 19, 24, 25, 27), the duration of post-traumatic amnesia (PTA) (5, 6, 9, 15, 19, 25), the occurrence (and/or duration) of LOC (5, 6, 14, 19, 22, 23) – measures available to the clinician early following TBI or by functional outcomes (e.g., neuropsychological test performance (4, 9, 15, 17, 19, 21, 22, 24, 26), behavioral tests, or other functional questionnaires (5, 6, 9, 10, 22, 26) (Tables 3 and 4). One study focused solely on severe TBI (4), while three were concerned with mild TBI only (6, 10, 16). Structural brain imaging was examined in relation to olfactory outcomes in a number of studies (12–14, 18, 24, 27) (Table 4).
In total, the studies referenced at least three olfactory constructs (sensitivity/threshold, identification, discrimination), 13 olfactory “instruments” [including olfactory event related potentials (ERPs)], administered in at least three different ways (unirhinal, birhinal, retronasal) and cited olfactory outcomes classified either categorically (e.g., normal/hyposmia/anosmia), as raw or scaled scores (relative to population norms) or according to change in olfactory performance over time. Olfactory “impairment” was, for the most part, defined by reference to a defined threshold or cut-off on an olfactory test. This approach also provided the opportunity to report a rate (incidence or prevalence) of impairment. In other studies, a significant mean reduction in olfactory performance in the post-TBI group relative to appropriate controls was reported, indicating impairment in some individuals. The University of Pennsylvania Smell Identification Test (UPSIT) was the olfactory instrument most commonly used (4, 8, 11, 17, 20–23, 28) followed by the Sniffin’ Sticks (6, 7, 10, 12) (Tables 5–8).
The interval between the TBI and olfactory testing ranged from 2 weeks (16) to many years (5, 17), and in some cases, it was not reported. Several studies compared different techniques for assessing post-TBI olfaction, including functional brain imaging or electrophysiological techniques (8, 12, 21). A single study employed qualitative techniques to focus on the functional consequences of post-TBI olfactory impairment (25) (Tables 5–8).
Main Findings
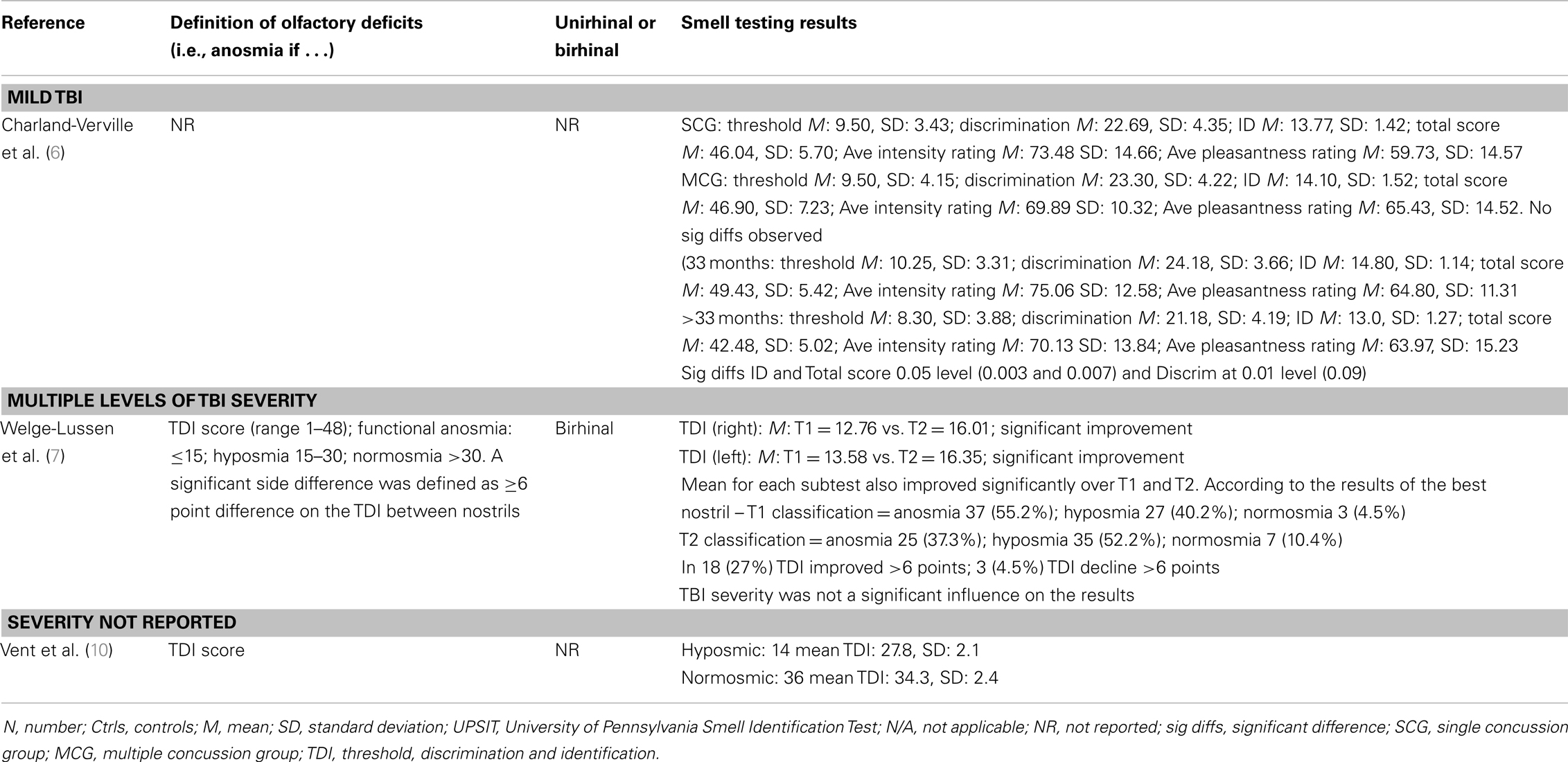
Of the studies that examined olfaction following mild TBI (6, 16) [or presumptive mild TBI (10)], the findings were mixed. Among athletes reporting concussion, there were no differences on olfactory testing relative to controls although, surprisingly, longer elapsed time since the most recent concussion was associated with significantly worse olfaction (6). This finding contrasted with those from longitudinal studies based on individuals with more severe TBI in which the overall trend was toward improvement in olfactory test performance over time, although post-TBI anosmia rarely if ever reverted to normal olfaction (7, 23). Among 111 individuals with mild TBI, 26% scored in an impaired range on an olfactory threshold test at 2 weeks post-injury (16). In a study of boxers including, apparently, many currently active in the sport, of whom approximately one-third had experienced at least one “knock out,” 28% were hyposmic, and as a group their olfactory performance was significantly worse than their matched controls (10). The results suggest that mild TBI or recurrent blows to the head might have an impact on olfaction, at least in the short term.
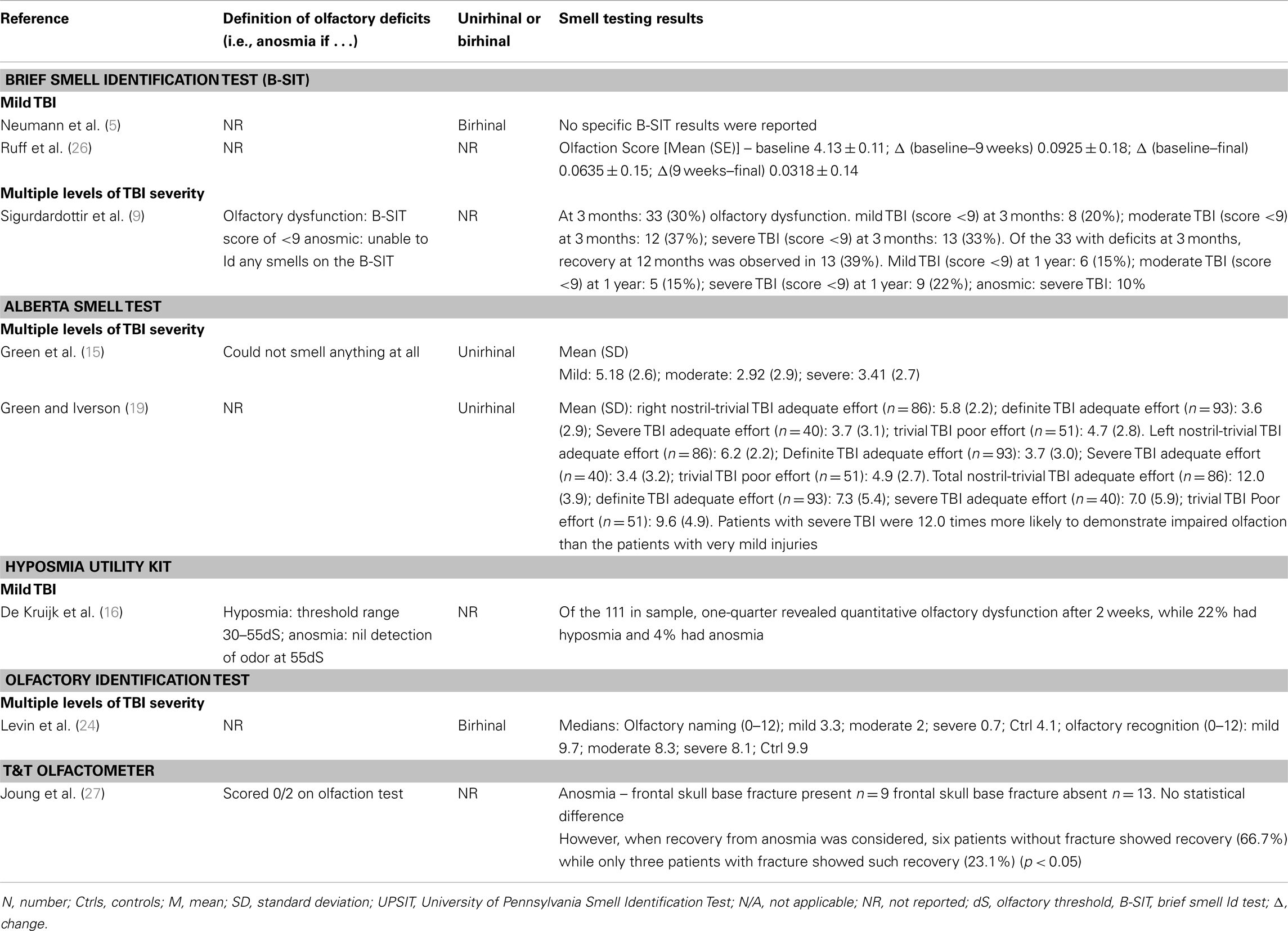
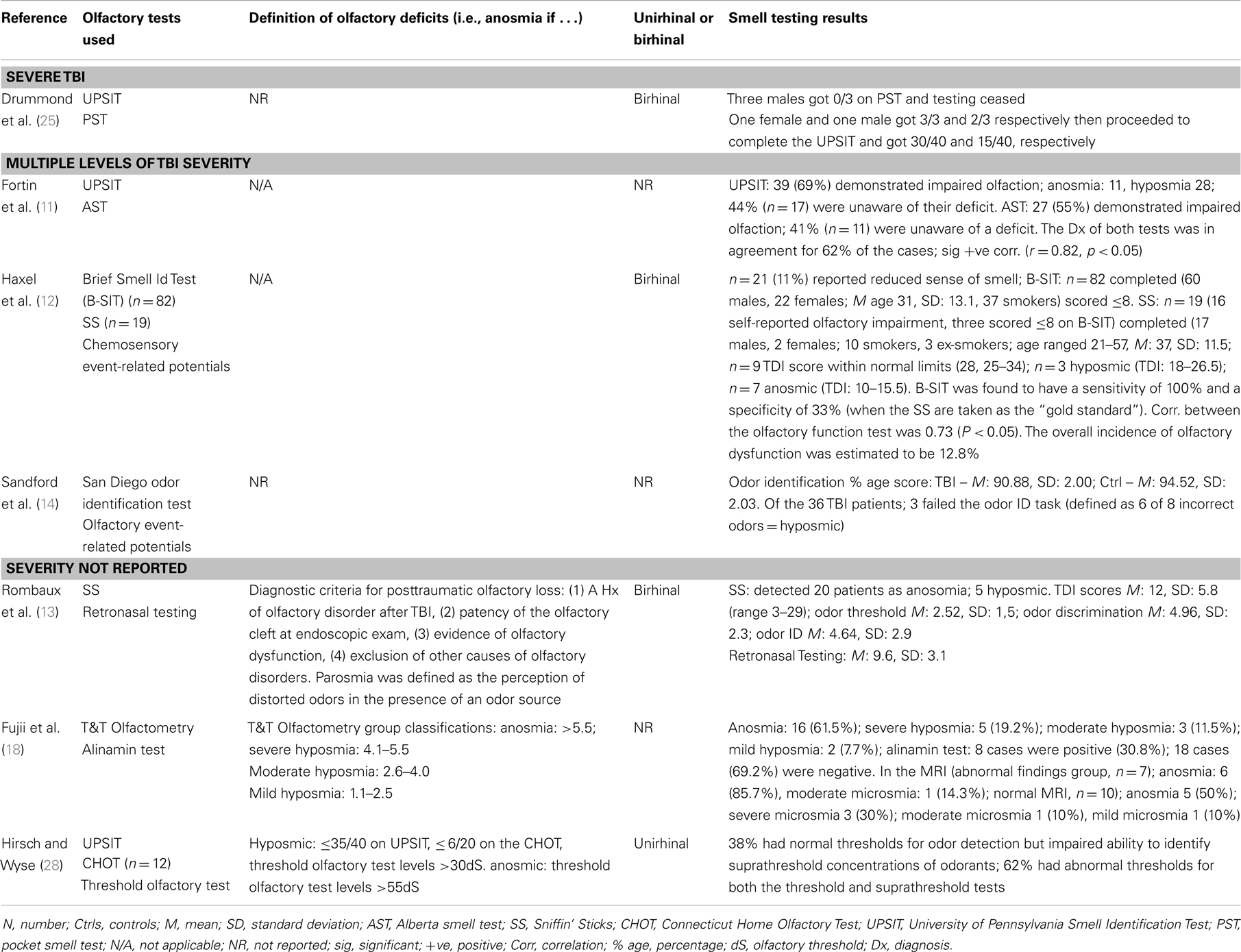
Of the studies that examined the relationship between olfactory function and severity of TBI, the findings were also mixed although for the most part they indicated an association (11, 14, 15, 17, 19, 24). A methodologically strong study by Levin et al. (24) included controls and the sample selection minimized bias; three different means of defining TBI severity (GCS, PTA, duration of LOC) were employed; and olfactory functioning was assessed using the Olfactory Identification Test (which includes naming and recognition trials). Olfactory tests were administered between 0.2 and 84 months after the injury and in all cases after resolution of PTA. Individuals with a GCS within the 13–15 range with mass lesions were combined with data from the GCS 9–12 group. Based on this assignment of severity, both the moderate and severe groups differed from the control group on olfactory tests and the trend for a decline in olfactory naming and recognition from mild to moderate and severe groups approached significance. Similar findings held when the duration of impaired consciousness or the duration of PTA was used to classify TBI severity. In each case, the mild group did not differ significantly from controls. Using the UPSIT, Callahan and Hinkebein found an inverse association with injury severity (GCS), but the relationship only emerged with the exclusion of individuals who failed tests of effort, many of whom had mild TBI and did relatively more poorly on olfactory testing (17). Sandford et al. divided pediatric patients with TBI into severity groups according to GCS, and assessed olfaction using the San Diego Children’s Odor Identification test (14). Only three children had olfactory dysfunction and as a group they had lower GCSs than those without. In another study, of 115 individuals examined at 3 months and 1 year post-TBI, the incidence of olfactory dysfunction was 22.3 and 13.5%, respectively [based on the Brief Smell Identification Test (B-SIT)] and there was no relationship of olfaction with TBI severity levels as defined by GCS (9). However, anosmia was more common in the severe TBI group, and performance on the B-SIT was significantly associated with verbal fluency performance, arguably a proxy for injury severity. A similar correspondence of olfactory test results with neuropsychological test performance was also evident in the studies of Green and colleagues (15, 19). Fortin et al. found that 69% of 49 individuals admitted to an outpatient’s rehabilitation program demonstrated impaired olfaction with no difference in rates according to TBI severity (11).
Estimates of the risk of olfactory dysfunction following a TBI are likely to vary in part due to factors, such as study-specific differences in the choice of olfactory tests and the cut-offs for “impairment,” different spectrums of TBI severity (and the means by which this was determined), and the interval of time since injury. Within the clinical studies reviewed in detail, the reported prevalence of olfactory dysfunction among cases with “mild” TBI was: 20% (9), 23% (15), 26% (16), 44% (17); among those with “moderate” TBI: 37% (9), 68.4% (17); with “moderate to severe” TBI: 49% (15), 56% (5); and with severe TBI:33% (9), 61% (17).
Haxel et al. attempted to minimize bias by using a stepped approach to the detection of olfactory dysfunction in a TBI-injured group identified through hospital records (12). Using a combination of subjective report, screening with the B-SIT, and definitive testing with the Sniffin’ Sticks, they estimated an overall prevalence of post-TBI olfactory dysfunction of 12.8% (12). In agreement with several other studies, they found significant rates of unawareness of olfactory dysfunction (5, 11, 17, 22) and evidence for increased risk of olfactory impairment associated with fractures to the base of skull and/or frontal hematomas (15, 20, 23, 24, 27). Hirsch and Wyse administered suprathreshold olfactory identification tests (the UPSIT and the Connecticut Home Olfactory Test) and olfactory threshold tests and defined two patterns of post-TBI dysomia (28). In one group, olfactory sensitivity was impaired but odor identification preserved. In the second group, scores were abnormal on both tests. They hypothesized that the first pattern would be consistent with peripheral pathology (e.g., olfactory nerves) while the second pattern might reflect pathology in central olfactory pathways or centers (28). Yousem et al. obtained high resolution magnetic resonance images of the olfactory bulbs and tracts and temporal lobes and related the findings to performance on the UPSIT, an odor memory test, and an olfactory threshold test, in cases with TBI and controls (20). The olfactory bulbs and tracts (89%), subfrontal lobes (61%), and temporal lobes (31%) of 36 patients showed the highest incidence of encephalomalacia and the left olfactory bulb and tract volumes showed a significant correlation with left and total UPSIT scores (20). The close proximity of the olfactory bulbs and tracts to the frontal lobes made dual pathology commonplace.
Several studies sought associations between post-TBI olfactory dysfunction and behavioral measures or other markers of neuropsychiatric dysfunction (5, 9, 22). In one study, individuals with post-TBI dysosmia performed more poorly on tests of affect recognition, emotional inference, and empathy (5). In another study, despite comparable GCS scores, the anosmic group displayed worse performance than the normosmic group on tests of memory and executive functioning and greater functional impairment as coded by the Disability Rating Scale (22).
Drummond, Douglas, and Oliver administered a semi-structured interview to five individuals who had sustained a severe TBI and had demonstrated olfactory dysfunction. All participants reported that the olfactory impairment had limited their ability to engage in specific activities including eating and enjoyment of food, food preparation, personal safety and hygiene, work, leisure, and personal relationships (25).
Discussion
The potential consequences of TBI are diverse. Injury severity varies enormously and numerous, complex pathophysiological mechanisms initiated by TBI alter the brain function acutely and beyond. Neuroanatomical and kinetic factors render the peripheral and central olfactory structures highly vulnerable to TBI-related damage, as reflected in the high prevalence of post-injury olfactory dysfunction reported here.
The results of this review confirm that post-TBI olfactory dysfunction is common. If persistent, it represents the loss of an important sensory function with potential functional consequences as eloquently outlined in the qualitative study cited immediately above (25). Remarkably, many individuals who suffer this complication appear to be unaware of it. Finally, its presence seems to signal an increased likelihood of adverse cognitive and other neuropsychiatric and functional outcomes. The implications of these findings are worth considering at the extremes of TBI severity.
Individuals who sustain severe TBI typically undergo brain imaging and receive medical services and rehabilitative efforts over extended periods. Optimally, neuropsychiatric and neuropsychological assessments are a routine component of this care. Ideally, this comprehensive and extended engagement should explicitly identify all TBI-related complications. The cognitive and behavioral changes of severe TBI are likely to diminish the capacity to deal effectively with risky situations such as, for example, fire or a gas leak. Identification before discharge of profound anosmia, which would further impair the early detection of such dangers could be justified as a clinical imperative. Severe TBI may lead to changes in social and occupational roles including, perhaps in males particularly, an increased role with food preparation with inherent risks for individuals with anosmia.
By contrast, “mild” TBI often attracts minimal if any clinical evaluation. Nevertheless, in some cases it may be far from benign. Intracerebral contusion and/or hemorrhage, as well as persisting cognitive or behavioral changes have been reported, albeit uncommonly (24). Several of the studies included in this review suggest that the identification of dysosmia/anosmia in a patient with mild TBI could serve to flag an increased likelihood of such unexpected complications (22, 24). In our view, more high quality studies evaluating olfaction and its correlates following mild TBI are needed. In light of the limited scope of the existing studies in this patient group, clarification of the evolution and clinical significance of any early post-injury olfactory changes would be invaluable.
The number and diversity of olfactory instruments and techniques available present a challenge for clinicians and researchers who wish to compare results across studies. It is beyond the scope of this review to venture specific recommendations regarding the “best” choice of instruments or the design of a “minimum data set” for future studies, even if the goal of “harmonization” is a worthy one. Financial resources, time, context, and the particular research question at hand clearly bring their own imperatives and necessarily influence such choices. As a general principle, however, the use of olfactory tests with good normative data, appropriate to the culture in which the study is to be conducted, is to be strongly encouraged. Ease of between-study comparisons would be enhanced if investigators were to consistently report both rates of olfactory impairment (based on well-defined criteria) and aggregated olfactory test score data (e.g., as expressed by group specific means ± standard deviation) as the latter measure does not necessarily easily convert to the former metric (i.e., prevalence of impairment).
As clinicians who routinely evaluate cognition, we are struck by the parallels between this activity and the conduct and interpretation of olfactory testing. The analogy holds in the context of TBI specifically. For both cognition and olfaction, the absence of symptoms cannot be relied upon to indicate intact functioning, or complaints to predict impairment on objective testing. Brevity of a screening instrument, while “convenient,” usually comes at the cost of reduced sensitivity but some testing is (almost always) better than none at all. Age, gender, and many extraneous factors may affect performance and, in the absence of a previous “premorbid” assessment, the etiological significance of a single abnormal test result may be difficult to determine, although the clinical context (and pre-test probability of impairment) are clearly relevant. Patients’ occupational and social responsibilities (viz. the cognitive or olfactory functioning challenges associated) might sensibly dictate who to prioritize for testing but such a strategy needs a background level of awareness as to the possibility of problems. We hope that this systematic review will make some contribution toward raising the awareness level in relation to olfactory dysfunction following TBI.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification test: a standardized microencapsulated test of olfactory function. Physiol Behav (1984) 32:489–502. doi:10.1016/0031-9384(84)90269-5
3. Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf SR. ‘Sniffin’ Sticks’: screening of olfactory performance. Rhinology (1996) 34:222–6.
4. Parma V, Straulino E, Zanatto D, Cantagallo A, Tirindelli R, Castiello U. Implicit olfactory abilities in traumatic brain injured patients. J Clin Exp Neuropsychol (2012) 34:977–88. doi:10.1080/13803395.2012.711811
5. Neumann D, Zupan B, Babbage DR, Radnovich AJ, Tomita M, Hammond F, et al. Affect recognition, empathy, and dysosmia after traumatic brain injury. Arch Phys Med Rehabil (2012) 93:1414–20. doi:10.1016/j.apmr.2012.03.009
6. Charland-Verville V, Lassonde M, Frasnelli J. Olfaction in athletes with concussion. Am J Rhinol Allergy (2012) 26:222–6. doi:10.2500/ajra.2012.26.3769
7. Welge-Lussen A, Hilgenfeld A, Meusel T, Hummel T. Long-term follow-up of posttraumatic olfactory disorders. Rhinology (2012) 50:67–72. doi:10.4193/Rhino11.141
8. Gerami H, Nemati S, Abbaspour F, Banan R. Brain single photon emission computed tomography in anosmic subjects after closed head trauma. Acta Med Iran (2011) 49:13–7.
9. Sigurdardottir S, Jerstad T, Andelic N, Roe C, Schanke AK. Olfactory dysfunction, gambling task performance and intracranial lesions after traumatic brain injury. Neuropsychology (2010) 24:504–13. doi:10.1037/a0018934
10. Vent J, Koenig J, Hellmich M, Huettenbrink KB, Damm M. Impact of recurrent head trauma on olfactory function in boxers: a matched pairs analysis. Brain Res (2010) 1320:1–6. doi:10.1016/j.brainres.2010.01.007
11. Fortin A, Lefebvre MB, Ptito M. Traumatic brain injury and olfactory deficits: the tale of two smell tests! Brain Inj (2010) 24:27–33. doi:10.3109/02699050903446815
12. Haxel BR, Grant L, Mackay-Sim A. Olfactory dysfunction after head injury. J Head Trauma Rehabil (2008) 23:407–13. doi:10.1097/01.HTR.0000341437.59627.ec
13. Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. Retronasal and orthonasal olfactory function in relation to olfactory bulb volume in patients with posttraumatic loss of smell. Laryngoscope (2006) 116:901–5. doi:10.1097/01.MLG.0000195291.36641.1E
14. Sandford AA, Davidson TM, Herrera N, Gilbert P, Magit AE, Haug K, et al. Olfactory dysfunction: a sequela of pediatric blunt head trauma. Int J Pediatr Otorhinolaryngol (2006) 70:1015–25. doi:10.1016/j.ijporl.2005.10.013
15. Green P, Rohling ML, Iverson GL, Gervais RO. Relationships between olfactory discrimination and head injury severity. Brain Inj (2003) 17:479–96. doi:10.1080/0269905031000070242
16. de Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Rutten J, Twijnstra A. Olfactory function after mild traumatic brain injury. Brain Inj (2003) 17:73–8. doi:10.1080/0269905021000010221
17. Callahan CD, Hinkebein JH. Assessment of anosmia after traumatic brain injury: performance characteristics of the University of Pennsylvania Smell Identification test. J Head Trauma Rehabil (2002) 17:251–6. doi:10.1097/00001199-200206000-00006
18. Fujii M, Fukazawa K, Takayasu S, Sakagami M. Olfactory dysfunction in patients with head trauma. Auris Nasus Larynx (2002) 29:35–40. doi:10.1016/S0385-8146(01)00118-3
19. Green P, Iverson GL. Effects of injury severity and cognitive exaggeration on olfactory deficits in head injury compensation claims. NeuroRehabilitation (2001) 16:237–43.
20. Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol (1999) 6:264–72. doi:10.1016/S1076-6332(99)80449-8
21. Geisler MW, Schlotfeldt CR, Middleton CB, Dulay MF, Murphy C. Traumatic brain injury assessed with olfactory event-related brain potentials. J Clin Neurophysiol (1999) 16:77–86. doi:10.1097/00004691-199901000-00008
22. Callahan CD, Hinkebein J. Neuropsychological significance of anosmia following traumatic brain injury. J Head Trauma Rehabil (1999) 14:581–7. doi:10.1097/00001199-199912000-00006
23. Doty RL, Yousem DM, Pham LT, Kreshak AA, Geckle R, Lee WW. Olfactory dysfunction in patients with head trauma. Arch Neurol (1997) 54:1131–40. doi:10.1001/archneur.1997.00550210061014
24. Levin HS, High WM, Eisenberg HM. Impairment of olfactory recognition after closed head injury. Brain (1985) 108:579–91. doi:10.1093/brain/108.3.579
25. Drummond M, Douglas J, Olver J. ‘If I haven’t got any smell. I’m out of work’: consequences of olfactory impairment following traumatic brain injury. Brain Inj (2013) 27:332–45. doi:10.3109/02699052.2012.750743
26. Ruff RL, Riechers RG, Wang XF, Piero T, Ruff SS. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev (2012) 49:1305–20. doi:10.1682/JRRD.2011.12.0251
Keywords: brain injury, olfaction, anosmia, trauma, review
Citation: Schofield PW, Moore TM and Gardner A (2014) Traumatic brain injury and olfaction: a systematic review. Front. Neurol. 5:5. doi: 10.3389/fneur.2014.00005
Received: 15 November 2013; Accepted: 09 January 2014;
Published online: 22 January 2014.
Edited by:
Yun Chen, US Army Medical Research Institute of Chemical Defense, USAReviewed by:
Charles W Wilkinson, University of Washington, USADavid Keyser, Uniformed Services University of the Health Sciences, USA
Paul Rapp, Uniformed Services University of the Health Sciences, USA
Copyright: © 2014 Schofield, Moore and Gardner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter William Schofield, Neuropsychiatry Service, Hunter New England Mental Health, PO BOX 833, Newcastle, NSW 2300, Australia e-mail: peter.schofield@hnehealth.nsw.gov.au
 Peter William Schofield
Peter William Schofield Tammie Maree Moore
Tammie Maree Moore Andrew Gardner
Andrew Gardner